삼당류: 두 판 사이의 차이
잔글편집 요약 없음 |
잔글편집 요약 없음 |
||
| 1번째 줄: | 1번째 줄: | ||
{{화합물 정보 |
{{화합물 정보 |
||
|이름 = |
|이름 = 뷰티르산 |
||
|그림 = |
|그림 = Butyric acid acsv.svg |
||
|그림크기 = |
|그림크기 = 200px |
||
|그림설명 = |
|그림설명 = |
||
|그림1 = Butyric-acid-3D-balls.png |
|||
|IUPAC = |
|||
|그림크기1 = 200px |
|||
|화학식 = C<sub>6</sub>H<sub>12</sub>O<sub>6</sub> |
|||
|IUPAC = Butanoic acid |
|||
|화학식 = C<sub>4</sub>H<sub>8</sub>O<sub>2</sub> |
|||
|분자식 = |
|분자식 = |
||
|별칭 = |
|별칭 = |
||
|CAS = |
|CAS = 107-92-6 |
||
|원자량 = |
|원자량 = |
||
|분자량 = |
|분자량 = 88.11 |
||
|녹는점 = |
|녹는점 = -5.1 |
||
|끓는점 = |
|끓는점 = 163.75 |
||
|밀도 = |
|밀도 = 0.9528 |
||
|용해도 = |
|용해도 = |
||
|용해성 = |
|용해성 = |
||
|상온상태 = |
|상온상태 = |
||
|상온색 = |
|상온색 = 무색 유성 액체 |
||
|기체 = |
|기체 = |
||
|기체1 = |
|기체1 = |
||
| 33번째 줄: | 35번째 줄: | ||
}} |
}} |
||
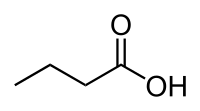
'''뷰티르산'''(<small>[[영어]]</small>: butyric acid) (그리스어 "βούτῡρον"에서 유래, "butter"를 의미함)은 계통명은 '''부탄산('''butanoic acid)이고, BTA로 약칭되며,<ref name=caslab>{{cite web|url = http://www.caslab.com/Butanoic-Acid.php5|title = Butanoic Acid|publisher = ALS Environmental|accessdate = 13 June 2014}}</ref> 화학식이 [[carbon|C]][[hydrogen|H]]<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>-[[carboxyl group|COOH]] 인 [[카복실산]]이다. 뷰티르산의 [[염]] 및 [[에스터]](에스테르)는 뷰티레이트(butyrate) 또는 부타노에이트(butanoate)로 알려져 있다. 뷰티르산은 [[우유]] 특히 [[염소 (동물)|염소]], [[양]] 및 [[아메리카들소|아메리카 들소]]의 젖, [[버터]], [[파마산 치즈]] 및 혐기성 [[발효]] 산물([[결장]] 및 [[액취증]] 포함)에서 발견된다. 또한 뷰티르산은 허쉬 공정에 의해 생산된 밀크 초콜릿에서 발견되거나, 허쉬 초콜릿의 맛을 모방하기 위해 첨가된 것으로 추측된다.<ref>{{cite news |url= https://www.nytimes.com/2008/02/13/dining/13chocolate.html |title=Dark may be king, but milk chocolate makes a move |first=Julia |last=Moskin |newspaper=The New York Times |date=13 February 2008 |accessdate=1 January 2016}}</ref> 뷰티르산은 사람의 [[구토]]물에 존재하며, 불쾌한 냄새를 낸다.<ref name="HMDB" /> 뷰티르산은 불쾌한 [[냄새]]와 콕 쏘는 [[맛]]을 가지며, [[에테르 (화학)|에테르]]와 유사한 약간 감미로운 뒷맛을 가진다. [[개]]와 같은 좋은 냄새 탐지 능력이 있는 [[포유류]]는 뷰티르산을 10ppb(parts per billion)로 탐지할 수 있는 반면에 사람은 10ppm(parts per million)이상의 농도에서 뷰티르산을 탐지할 수 있다. |
|||
'''갈락토스'''([[영어|<small>영어</small>]]: galactose)는 [[육탄당]](알도헥소스)의 하나로 [[글루코스|글루코스(포도당)]]만큼 단맛이 나며, 설탕의 30%정도의 단맛을 내는 [[단당류]]이다.<ref>{{Cite book|url=https://books.google.com/books?id=db5QAwAAQBAJ&pg=PA264|title=Optimising Sweet Taste in Foods|last=Spillane|first=W. J.|date=2006-07-17|publisher=Woodhead Publishing|year=|isbn=9781845691646|location=|pages=264|language=en}}</ref> 갈락토스는 글루코스의 C-4 [[에피머]]이다.<ref>{{Cite book|url=https://books.google.com/books?id=QOoPlLgN5UMC&pg=PA43|title=Organic Reactions Stereochemistry And Mechanism (Through Solved Problems)|last=Kalsi|first=P. S.|date=2007|publisher=New Age International|year=|isbn=9788122417661|location=|pages=43|language=en}}</ref> [[갈락탄]]은 [[헤미셀룰로스]]에서 발견되는 갈락토스의 [[중합체]]이고, 가수분해에 의해 갈락토스로 전환될 수 있다.<ref>{{Cite book|url=https://books.google.de/books?id=GDuy5zL6eRAC&pg=PA78|title=The Antibodies|last=Zanetti|first=Maurizio|last2=Capra|first2=Donald J.|date=2003-09-02|publisher=CRC Press|year=|isbn=9780203216514|location=|pages=78|language=en}}</ref> |
|||
뷰티르산은 1814년 프랑스의 화학자 미셀 외젠 슈프뢰이(Michel Eugène Chevreul)에 의해 불순한 형태로 처음 관찰되었다. 1818년까지 슈브외이는 뷰티르산을 특징짓기 위해 충분하게 정제했다. 그러나 슈브뢰이는 뷰티르산에 대한 초기 연구를 발표하지 않았고, 대신 프랑스 파리에 있는 과학 아카데미에 원고 형태로 연구 결과물을 맡겼다. 또한 프랑스의 화학자인 앙리 브라코노(Henri Braconnot)도 버터의 성분을 연구하고 연구 결과를 발표했는데 이것이 우선권에 대한 논쟁으로 이어졌다. 1815년 초에 슈브뢰이는 버터 냄새의 원인이 되는 물질을 발견했다고 주장했다.<ref>Chevreul (1815) [https://books.google.com/books?id=tZU5AAAAcAAJ&pg=PA73#v=onepage&q&f=false "Lettre de M. Chevreul à MM. les rédacteurs des Annales de chimie"] (Letter from Mr. Chevreul to the editors of the Annals of Chemistry), ''Annales de chimie'', '''94''' : 73–79; in a footnote spanning pages 75–76, he mentions that he had found a substance that is responsible for the smell of butter.</ref> 1817년에 슈브뢰이는 뷰티르산의 성질에 관한 연구 결과를 발표하고, 뷰티르산의 이름을 지었다.<ref>Chevreul (1817) [https://books.google.com/books?id=y1E3AAAAYAAJ&pg=PA79#v=onepage&q&f=false "Extrait d'une lettre de M. Chevreul à MM. les Rédacteurs du Journal de Pharmacie"] (Extract of a letter from Mr. Chevreul to the editors of the Journal of Pharmacy), ''Journal de Pharmacie et des sciences accessoires'', '''3''' : 79–81. On p. 81, he named butyric acid: ''"Ce principe, que j'ai appelé depuis acid butérique, … "'' (This principle [i.e., constituent], which I have since named "butyric acid", … )</ref> 그러나, 1823년에 이르러서야 뷰티르산의 특성이 자세히 밝혀졌다.<ref>E. Chevreul, ''Recherches chimiques sur les corps gras d'origine animale'' [Chemical researches on fatty substances of animal origin] (Paris, France: F.G. Levrault, 1823), [https://books.google.com/books?id=r46rnl27h70C&pg=PA115#v=onepage&q&f=false pages 115–133].</ref> 뷰티르산이란 이름은 처음 발견된 물질인 ''butyrum'' (또는 ''buturum'')이라는 버터의 라틴어 단어에서 유래되었다. |
|||
==어원== |
|||
갈락토스라는 이름은 19세기 중엽 찰스 바이스만(Charles Weissman)에 의해 명명되었으며,<ref>{{cite web|title=Charles Weismann in the 1940 Census {{!}} Ancestry|url=https://www.ancestry.com/1940-census/usa/New-York/Charles-Weismann_86gdp|website=www.ancestry.com|accessdate=26 December 2017|language=en}}</ref> 그리스어 "''galaktos''(milk)" 와 당을 뜻하는 일반적인 화학 접미사인 "''-ose''" 에서 유래되었다.<ref>{{cite web|last1=Bhat|first1=Paike Jayadeva|title=Galactose Regulon of Yeast: From Genetics to Systems Biology|url=https://books.google.co.in/books?id=ofEx-CsVlIAC&pg=PA127&lpg=PA127&dq=galactose+is+coined+by&source=bl&ots=MB7yRs_sqW&sig=N0_Bp8dmRdCDQBMTS3wVS9sOayw&hl=en&sa=X&ved=0ahUKEwjI-4ru7KfYAhWMPI8KHR9pCzMQ6AEIOTAC#v=onepage&q=galactose%20is%20coined%20by&f=false|publisher=Springer Science & Business Media|accessdate=26 December 2017|language=en|date=2 March 2008}}</ref> |
|||
==화학== |
|||
==구조와 이성질체== |
|||
뷰티르산은 동물성 지방에서 [[에스터]]의 형태로 발견되는 [[지방산]]이다. 뷰티르산의 [[트라이글리세라이드]]는 버터의 3~4%를 차지한다. 버터가 산패할 때, [[가수 분해|가수분해]]에 의해 글리세라이드로부터 뷰티르산이 유리되어 불쾌한 냄새가 난다. 뷰티르산은 짧은 사슬 지방산이라고 불리는 지방산 하위 그룹의 주요 구성원이다. 뷰티르산은 염기와 강력한 산화제와 반응하여 많은 금속을 공격하는 중-강산이다.<ref name="inchem1">[http://www.inchem.org/documents/icsc/icsc/eics1334.htm ICSC 1334 – BUTYRIC ACID]. Inchem.org (23 November 1998). Retrieved on 2014-03-31.</ref> |
|||
갈락토스는 사슬형과 고리형으로 존재한다. 사슬형은 사슬의 끝부분에 [[카보닐기]]를 갖는다. |
|||
뷰티르산은 [[물]], [[에탄올]] 및 [[에테르 (화학)|에테르]]에 쉽게 용해되는 유성의 무색 액체이며 [[염화 칼슘]]과 같은 염으로 포화되어 액상으로부터 분리될 수 있다. It is oxidized to [[carbon dioxide]] and [[acetic acid]] using [[potassium dichromate]] and [[sulfuric acid]], while alkaline [[potassium permanganate]] oxidizes it to carbon dioxide. The calcium salt, Ca(C<sub>4</sub>H<sub>7</sub>O<sub>2</sub>)<sub>2</sub>·H<sub>2</sub>O, is less soluble in hot water than in cold. |
|||
4개의 이성질체는 고리형인데 그 중 2개는 [[피라노스]] 고리이고, 2개는 [[푸라노스]] 고리이다. 갈락토푸라노스는 세균, 균류 및 원생동물에서 발견되고,<ref>Nassau et al. [http://jb.asm.org/cgi/reprint/178/4/1047.pdf Galactofuranose Biosynthesis in Escherichia coli K-12:...] Journal of Bacteriology, Feb. 1996, p. 1047–1052</ref> 외향고리 1,2-디올을 통해 척삭동물의 면역 렉틴으로 추정되는 인텔렉틴(intelectin)에 의해 인식된다. 사슬형에서 고리형으로의 변화가 사슬의 카보닐 부위에서 새로운 입체 중심의 생성을 포함하기 때문에 고리형에서 α, β로 명명된 2개의 [[아노머]]가 있다. β형에서 [[하이드록시기]]는 수평 방향에 위치하는 반면 α형에서는 하이드록시기가 축 방향에 위치한다.<ref name="Ophardt, C. Galactose">[http://elmhcx9.elmhurst.edu/~chm/vchembook/543galactose.html Ophardt, C. Galactose]</ref> |
|||
Butyric acid has a [[structural isomer]] called [[isobutyric acid]] (2-methylpropanoic acid). |
|||
{| class="wikitable" style="text-align:center" |
|||
|- class="hintergrundfarbe8" |
|||
===Safety=== |
|||
!colspan="3"| <small>D</small>-갈락토스의 구조 |
|||
|- class="hintergrundfarbe5" |
|||
Personal protective equipment such as rubber or PVC gloves, protective eye goggles, and chemical-resistant clothing and shoes are used to minimize risks when handling butyric acid. |
|||
! 골격구조식 |
|||
!colspan="2"| 하워드 투영식 |
|||
Inhalation of butyric acid may result in soreness of throat, coughing, a burning sensation, and laboured breathing. Ingestion of the acid may result in abdominal pain, shock, and collapse. Physical exposure to the acid may result in pain, blistering and skin burns, while exposure to the eyes may result in pain, severe deep burns and loss of vision.<ref name="inchem1"/> |
|||
|- class="hintergrundfarbe2" |
|||
|rowspan="2"| [[파일:D-Galactose Keilstrich.svg|100px]] |
|||
==Production== |
|||
| [[파일:Alpha-D-Galactofuranose.svg|130px]]<br />α-<small>D</small>-갈락토푸라노스 |
|||
| [[파일:Beta-D-Galactofuranose.svg|130px]]<br />β-<small>D</small>-갈락토푸라노스 |
|||
It is industrially prepared by the fermentation of [[sugar]] or [[starch]], brought about by the addition of putrefying [[cheese]], with [[calcium carbonate]] added to neutralize the acids formed in the process. The butyric fermentation of starch is aided by the direct addition of ''[[Bacillus subtilis]]''. Salts and esters of the acid are called [[butyrate]]s or butanoates. |
|||
|- class="hintergrundfarbe2" |
|||
| [[파일:Alpha-D-Galactopyranose.svg|90px]]<br />α-<small>D</small>-갈락토피라노스 |
|||
Butyric acid or fermentation butyric acid is also found as a hexyl [[ester]] [[hexyl butyrate]] in the oil of ''Heracleum giganteum'' (a type of [[Heracleum (plant)|hogweed]]) and as the octyl ester [[octyl butyrate]] in [[parsnip]] (''Pastinaca sativa''); it has also been noticed in [[skin flora]] and perspiration. |
|||
| [[파일:Beta-D-Galactopyranose.svg|90px]]<br />β-<small>D</small>-갈락토피라노스 |
|||
==Uses== |
|||
Butyric acid is used in the preparation of various butyrate esters. Low-molecular-weight esters of butyric acid, such as [[methyl butyrate]], have mostly pleasant aromas or tastes. As a consequence, they are used as food and perfume additives. It is also used as an animal feed supplement due to the ability to reduce pathogenic bacterial colonization.<ref>[http://ps.fass.org/cgi/reprint/84/12/1851.pdf Supplementation of Coated Butyric Acid in the Feed Reduces Colonization and Shedding of Salmonella in Poultry]. Ps.fass.org. Retrieved on 31 March 2014.</ref> It is an approved food flavoring in the EU FLAVIS database (number 08.005). |
|||
Due to its powerful odor, it has also been used as a fishing bait additive.<ref>[http://www.nutrabaits.net/freezer.html Freezer Baits], nutrabaits.net</ref> Many of the commercially available flavors used in [[Common carp|carp]] (''Cyprinus carpio'') baits use butyric acid as their ester base; however, it is not clear whether fish are attracted by the butyric acid itself or the substances added to it. Butyric acid was, however, one of the few organic acids shown to be palatable for both [[tench]] and [[bitterling]].<ref>{{Cite journal | doi = 10.1046/j.1467-2979.2003.00121.x | last1 = Kasumyan | first1 = A.O. | last2 = Døving | first2 = K.B. | year = 2003 | title = Taste preferences in fishes | url = | journal = Fish and Fisheries | volume = 4 | issue = 4| pages = 289–347 | name-list-format = vanc}}</ref> |
|||
The substance has also been used as a [[stink bomb]] by [[Sea Shepherd Conservation Society]] to disrupt Japanese whaling crews,<ref>[http://www.newser.com/story/80755/japanese-whalers-injured-by-acid-firing-activists.html Japanese Whalers Injured by Acid-Firing Activists], newser.com, 10 February 2010</ref> as well as by anti-abortion protesters to disrupt abortion clinics.<ref>[http://www.prochoice.org/about_abortion/violence/butyric_acid.asp National Abortion Federation, HISTORY OF VIOLENCE Butyric Acid Attacks] {{webarchive|url=https://web.archive.org/web/20100613053253/http://prochoice.org/about_abortion/violence/butyric_acid.asp |date=13 June 2010 }}. Prochoice.org. Retrieved on 31 March 2014.</ref> |
|||
Butyric acid, along with acetic acid, can be reacted with cellulose to produce the organic ester [[Cellulose#Derivatives|cellulose acetate butyrate]] (CAB), which is used in a wide variety of tools, parts, and coatings and is more resistant to degradation than [[cellulose acetate]].<ref>{{cite book|last1=Lokensgard|first1=Erik|title=Industrial Plastics: Theory and Applications|date=2015|publisher=Cengage Learning|edition=6th}}</ref> However, CAB can degrade with exposure to heat and moisture, releasing butyric acid.<ref>{{cite news|last1=Williams|first1=R. Scott|title=Care of Plastics: Malignant plastics|url=http://cool.conservation-us.org/waac/wn/wn24/wn24-1/wn24-102.html|accessdate=29 May 2017|work=WAAC Newsletter|issue=Vol. 24, No. 1|publisher=Conservation OnLine}}</ref> This process is sometimes observed in the unpleasant, vomit-like odor of aging screwdrivers and other hand tools.<ref>{{cite web|title=Why screwdriver handles smell like vomit and make your tongue numb.|url=http://www.garagejournal.com/forum/showthread.php?t=289078&showall=1|website=The Garage Journal|accessdate=29 May 2017}}</ref> |
|||
== Biochemistry == |
|||
=== Microbial biosynthesis === |
|||
{{missing information|section|date=May 2015|2 additional metabolic pathways: [http://mbio.asm.org/content/5/2/e00889-14/F1.expansion.html]}} |
|||
Butyrate is produced as end-product of a fermentation process solely performed by [[Obligate anaerobe|obligate]] [[anaerobic organism|anaerobic]] [[bacteria]]. Fermented [[Kombucha]] "tea" includes butyric acid as a result of the fermentation. This fermentation pathway was discovered by [[Louis Pasteur]] in 1861. Examples of butyrate-producing [[species]] of bacteria: |
|||
*''[[Clostridium butyricum]]'' |
|||
*''[[Clostridium kluyveri]]'' |
|||
*''Clostridium pasteurianum'' |
|||
*''[[Faecalibacterium prausnitzii]]'' |
|||
*''[[Fusobacterium nucleatum]]'' |
|||
*''Butyrivibrio fibrisolvens'' |
|||
*''Eubacterium limosum'' |
|||
The pathway starts with the [[glycolysis|glycolytic]] cleavage of [[glucose]] to two [[molecule]]s of [[pyruvate]], as happens in most organisms. Pyruvate is then [[oxidation|oxidized]] into [[acetyl coenzyme A]] using a unique mechanism that involves an [[enzyme]] system called [[pyruvate:ferredoxin oxidoreductase]]. Two molecules of [[carbon dioxide]] (CO<sub>2</sub>) and two molecules of elemental [[hydrogen]] (H<sub>2</sub>) are formed as waste products from the cell. Then, |
|||
{| class="wikitable" |
|||
|- |
|||
!Action!!Responsible enzyme |
|||
|- |
|||
| Acetyl coenzyme A converts into [[acetoacetyl coenzyme A]] || [[acetyl-CoA-acetyl transferase]] |
|||
|- |
|||
| Acetoacetyl coenzyme A converts into [[β-hydroxybutyryl CoA]] || [[β-hydroxybutyryl-CoA dehydrogenase]] |
|||
|- |
|||
| β-hydroxybutyryl CoA converts into [[crotonyl CoA]] || [[crotonase]] |
|||
|- |
|||
| Crotonyl CoA converts into [[butyryl CoA]] (CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>C=O-CoA) || [[butyryl CoA dehydrogenase]] |
|||
|- |
|||
| A [[phosphate]] group replaces CoA to form [[butyryl phosphate]] || [[phosphobutyrylase]] |
|||
|- |
|||
| The phosphate group joins [[adenosine diphosphate|ADP]] to form [[adenosine triphosphate|ATP]] and [[butyrate]] || [[butyrate kinase]] |
|||
|} |
|} |
||
ATP is produced, as can be seen, in the last step of the fermentation. Three molecules of ATP are produced for each glucose molecule, a relatively high yield. The balanced equation for this fermentation is |
|||
==젖당과의 관계== |
|||
:C<sub>6</sub>H<sub>12</sub>O<sub>6</sub> → C<sub>4</sub>H<sub>8</sub>O<sub>2</sub> + 2 CO<sub>2</sub> + 2 H<sub>2</sub> |
|||
갈락토스는 단당류이며 탈수축합반응을 통해 단당류인 포도당과 결합하여 [[이당류]]인 [[락토스|락토스(젖당)]]을 생성한다. [[락테이스]]와 β-갈락토시데이스는 젖당을 포도당과 갈락토스로 [[가수 분해|가수분해]]하는 반응을 촉매한다. β-갈락토시데이스는 대장균(''Escherichia coli'')의 ''lac'' 오페론에 의해 만들어진다. |
|||
Several species form [[acetone]] and [[n-Butanol|''n''-butanol]] in an alternative pathway, which starts as butyrate fermentation. Some of these species are: |
|||
자연에서 젖당은 주로 [[우유]] 및 [[유제품]]에서 발견된다. 따라서, 낙농 유래 성분으로 제조된 다양한 식품은 젖당을 함유할 수 있다.<ref>{{cite web |url= http://digestive.niddk.nih.gov/ddiseases/pubs/lactoseintolerance/ |title=Lactose Intolerance – National Digestive Diseases Information Clearinghouse |last=Staff <!-- Verified: No author provided on page. --> |work=digestive.niddk.nih.gov |date=June 2009 |accessdate=January 11, 2014}}</ref> 갈락토스를 포도당으로 전환시키는 갈락토스 대사는 를루아르(Leloir) 경로로 알려진 메커니즘에서 3가지 주요 효소들에 의해 수행된다. 효소들을 [[물질대사|대사]] 경로 순으로 나열하면 갈락토카이네이스(GALK), 갈락토스-1-인산 유리딜 전이효소(GALT), UDP-갈락토스-4’-에피머화효소(GALE)이다. |
|||
*''[[Clostridium acetobutylicum]]'', the most prominent acetone and propianol producer, used also in industry |
|||
*''[[Clostridium beijerinckii]]'' |
|||
*''[[Clostridium tetanomorphum]]'' |
|||
*''[[Clostridium aurantibutyricum]]'' |
|||
These bacteria begin with butyrate fermentation, as described above, but, when the [[pH]] drops below 5, they switch into butanol and acetone production to prevent further lowering of the pH. Two molecules of butanol are formed for each molecule of acetone. |
|||
사람의 [[모유 수유|수유]] 과정시 [[젖샘]]에서 젖당을 분비할 수 있도록 포도당은 갈락토스로 전환된다. 그러나 [[모유]]에서 대부분의 젖당은 혈액에서 공급된 갈락토스로부터 합성되며, 단지 35±6%만이 새로 합성된 갈락토스로부터 만들어진다.<ref name=sunehag>{{cite journal |vauthors=Sunehag A, Tigas S, Haymond MW |title=Contribution of plasma galactose and glucose to milk lactose synthesis during galactose ingestion |journal=J. Clin. Endocrinol. Metab. |volume=88 |issue=1 |pages=225–9 |date=January 2003 |pmid=12519857 |doi= 10.1210/jc.2002-020768|url=http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=12519857}}</ref> 글리세롤도 젖샘의 갈락토스 생산에 일부 기여한다.<ref>{{cite journal |vauthors=Sunehag AL, Louie K, Bier JL, Tigas S, Haymond MW |title=Hexoneogenesis in the human breast during lactation |journal=J. Clin. Endocrinol. Metab. |volume=87 |issue=1 |pages=297–301 |date=January 2002 |pmid=11788663 |doi= 10.1210/jc.87.1.297|url=http://jcem.endojournals.org/cgi/pmidlookup?view=long&pmid=11788663}}</ref> |
|||
The change in the pathway occurs after acetoacetyl CoA formation. This intermediate then takes two possible pathways: |
|||
==대사== |
|||
*acetoacetyl CoA → acetoacetate → acetone |
|||
{| class="toccolours collapsible collapsed" width="100%" style="text-align:left" |
|||
*acetoacetyl CoA → butyryl CoA → [[butyraldehyde]] → butanol |
|||
! 일반적인 단당류의 대사 및 포도당의 대사 과정 |
|||
Highly-fermentable fiber residues, such as those from [[resistant starch]], [[oat bran]], [[pectin]], and [[guar]] are transformed by [[gut flora|colonic bacteria]] into [[short-chain fatty acid]]s (SCFA) including butyrate, producing more SCFA than less fermentable fibers such as [[cellulose]]s.<ref name="lupton">{{cite journal | vauthors = Lupton JR | title = Microbial degradation products influence colon cancer risk: the butyrate controversy | journal = The Journal of Nutrition | volume = 134 | issue = 2 | pages = 479–82 | date = February 2004 | pmid = 14747692 | url = http://jn.nutrition.org/cgi/content/full/134/2/479 }}</ref> One study found that resistant starch consistently produces more butyrate than other types of [[dietary fiber]].<ref>{{cite journal | vauthors = Cummings JH, Macfarlane GT, Englyst HN | title = Prebiotic digestion and fermentation | journal = The American Journal of Clinical Nutrition | volume = 73 | issue = 2 Suppl | pages = 415S-420S | date = February 2001 | pmid = 11157351 }}</ref> The production of SCFA from fibers in [[ruminant]] animals such as cattle is responsible for the butyrate content of milk and butter.<ref>{{cite journal | vauthors = Grummer RR | title = Effect of feed on the composition of milk fat | journal = Journal of Dairy Science | volume = 74 | issue = 9 | pages = 3244–57 | date = September 1991 | pmid = 1779073 | doi = 10.3168/jds.S0022-0302(91)78510-X | url = http://download.journals.elsevierhealth.com/pdfs/journals/0022-0302/PIIS002203029178510X.pdf }}</ref> |
|||
Fructans are another source of prebiotic soluble dietary fibers. They are often found in the soluble fibers of foods which are high in [[Low-sulfur diet|sulfur]], such as the [[Allium]] and [[Cruciferous vegetables|Cruciferous]] vegetables. [[Fructan#Fructan content of various foods|Sources of fructans]] include [[wheat]] (although some wheat strains such as [[spelt]] contain lower amounts),<ref>{{cite web|url=http://www.med.monash.edu/cecs/gastro/fodmap/diet-and-ibs.html#5|title=Frequently asked questions in the area of diet and IBS|last=webmed|website=www.med.monash.edu|access-date=24 March 2016}}</ref> [[rye]], [[barley]], [[onion]], [[garlic]], [[Jerusalem artichoke|Jerusalem]] and [[globe artichoke]], [[asparagus]], [[beetroot]], [[chicory]], [[Dandelion|dandelion leaves]], [[leek]], [[radicchio]], the white part of [[spring onion]], [[broccoli]], [[brussels sprouts]], [[cabbage]], [[fennel]] and [[Prebiotic (nutrition)|prebiotics]] such as fructooligosaccharides ([[Fructooligosaccharide|FOS]]), [[oligofructose]] and [[inulin]].<ref>{{Cite journal|last=Gibson|first=Peter R.|last2=Shepherd|first2=Susan J.|date=1 February 2010|title=Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach|journal=Journal of Gastroenterology and Hepatology|volume=25|issue=2|pages=252–258|doi=10.1111/j.1440-1746.2009.06149.x|issn=1440-1746|pmid=20136989}}</ref><ref>{{Cite journal|last=Gibson|first=Peter R.|last2=Varney|first2=Jane|last3=Malakar|first3=Sreepurna|last4=Muir|first4=Jane G.|date=1 May 2015|title=Food components and irritable bowel syndrome|journal=Gastroenterology|volume=148|issue=6|pages=1158–1174.e4|doi=10.1053/j.gastro.2015.02.005|issn=1528-0012|pmid=25680668}}</ref> While many of these foods lack in butyrate production compared to resistant starch, they do have a number of benefits. They generally possess a low [[glycemic index]] which appeals well to diabetics. They also appeal to those on the [[ketogenic diet]] who benefit from [[beta-hydroxybutyric acid]], which is a HDAC inhibitor which can cross the blood brain barrier and be used as fuel in the [[Mitochondrion|mitochondria]] of brain cells.<ref>{{Cite journal|last=Owen|first=O. E.|last2=Morgan|first2=A. P.|last3=Kemp|first3=H. G.|last4=Sullivan|first4=J. M.|last5=Herrera|first5=M. G.|last6=Cahill|first6=G. F.|date=1 October 1967|title=Brain metabolism during fasting|journal=The Journal of Clinical Investigation|volume=46|issue=10|pages=1589–1595|doi=10.1172/JCI105650|issn=0021-9738|pmc=292907|pmid=6061736}}</ref> Other HDAC inhibitors in these butyrate producing foods are [[sulforaphane]],<ref>{{Cite journal|last=Ho|first=Emily|last2=Clarke|first2=John D.|last3=Dashwood|first3=Roderick H.|date=1 December 2009|title=Dietary Sulforaphane, a Histone Deacetylase Inhibitor for Cancer Prevention|journal=The Journal of Nutrition|volume=139|issue=12|pages=2393–2396|doi=10.3945/jn.109.113332|issn=0022-3166|pmc=2777483|pmid=19812222}}</ref> which has promise in inhibiting human breast cancer cells.<ref>{{Cite journal|last=Pledgie-Tracy|first=Allison|last2=Sobolewski|first2=Michele D.|last3=Davidson|first3=Nancy E.|date=1 March 2007|title=Sulforaphane induces cell type–specific apoptosis in human breast cancer cell lines|url=http://mct.aacrjournals.org/content/6/3/1013|journal=Molecular Cancer Therapeutics|language=en|volume=6|issue=3|pages=1013–1021|doi=10.1158/1535-7163.MCT-06-0494|issn=1535-7163|pmid=17339367}}</ref> Sulforaphane has also been shown to promote hair growth in mice,<ref>{{Cite journal|last=Sasaki|first=Mari|last2=Shinozaki|first2=Shohei|last3=Shimokado|first3=Kentaro|date=25 March 2016|title=Sulforaphane promotes murine hair growth by accelerating the degradation of dihydrotestosterone|journal=Biochemical and Biophysical Research Communications|volume=472|issue=1|pages=250–254|doi=10.1016/j.bbrc.2016.02.099|issn=1090-2104|pmid=26923074}}</ref> it contains compounds which prevent ulcers,<ref>{{Cite journal|last=Moon|first=Joon-Kwan|last2=Kim|first2=Jun-Ran|last3=Ahn|first3=Young-Joon|last4=Shibamoto|first4=Takayuki|date=9 June 2010|title=Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli ( Brassica oleracea L.) sprouts|journal=Journal of Agricultural and Food Chemistry|volume=58|issue=11|pages=6672–6677|doi=10.1021/jf1003573|issn=1520-5118|pmid=20459098}}</ref> and helps with cognitive function in rats.<ref>{{Cite journal|last=Dash|first=Pramod K.|last2=Zhao|first2=Jing|last3=Orsi|first3=Sara A.|last4=Zhang|first4=Min|last5=Moore|first5=Anthony N.|date=28 August 2009|title=Sulforaphane improves cognitive function administered following traumatic brain injury|journal=Neuroscience Letters|volume=460|issue=2|pages=103–107|doi=10.1016/j.neulet.2009.04.028|issn=1872-7972|pmc=2700200|pmid=19515491}}</ref> It is good to note that sulforaphane in broccoli is destroyed if prepared improperly.<ref>{{Cite journal|last=Ghawi|first=Sameer Khalil|last2=Methven|first2=Lisa|last3=Niranjan|first3=Keshavan|title=The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba)|url=https://www.researchgate.net/publication/235628559_The_potential_to_intensify_sulforaphane_formation_in_cooked_broccoli_Brassica_oleracea_var_italica_using_mustard_seeds_Sinapis_alba|journal=Food Chemistry|volume=138|issue=2–3|pages=1734–1741|doi=10.1016/j.foodchem.2012.10.119}}</ref> [[Diallyl disulfide]] found in the fructans containing garlic has been shown to reduce chemical toxicity and carcinogenesis in rodents,<ref>{{Cite journal|last=Yang|first=C. S.|last2=Chhabra|first2=S. K.|last3=Hong|first3=J. Y.|last4=Smith|first4=T. J.|date=1 March 2001|title=Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic|journal=The Journal of Nutrition|volume=131|issue=3s|pages=1041S–5S|issn=0022-3166|pmid=11238812}}</ref> and shows synergestic benefits with butyrate when it comes to inhibiting the growth of human cancer tumor cells in the colon.<ref>{{Cite journal|last=Sundaram|first=Sujatha G.|last2=Milner|first2=John A.|date=1 April 1996|title=Diallyl disulfide induces apoptosis of human colon tumor cells|url=http://carcin.oxfordjournals.org/content/17/4/669|journal=Carcinogenesis|language=en|volume=17|issue=4|pages=669–673|doi=10.1093/carcin/17.4.669|issn=0143-3334|pmid=8625476}}</ref> |
|||
==Pharmacology== |
|||
{| class="wikitable unsortable" style="text-align:center; float:right" |
|||
|+ Human enzyme and GPCR binding<ref name="IUPHAR">{{cite web|title=Butyric acid|url=http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=biology&ligandId=1059|website=IUPHAR|publisher=IUPHAR/BPS Guide to PHARMACOLOGY|accessdate=23 May 2015}}</ref><ref name="BindingDB">{{cite web|title=butanoic acid, 4 and Sodium; butyrate|url=http://www.bindingdb.org/bind/searchby_smiles.jsp?submit=Search&startPg=0&Increment=15&SearchType=3&smilesStr=CCCC%28%5BO-%5D%29%3DO&molfile=CCCC%28%5BO-%5D%29%3DO&Similarity=.99|website=BindingDB|publisher=The Binding Database|accessdate=23 May 2015}}</ref> |
|||
! Inhibited enzyme !! [[IC50|IC<sub>50</sub>]] ({{abbr|nM|nanomolar}}) !! Entry note |
|||
|- |
|- |
||
| [[HDAC1]] || 16,000 || |
|||
|[[File:Metabolism of common monosaccharides, and related reactions.png|none|1000px]] |
|||
|- |
|||
| [[HDAC2]] || 12,000 || |
|||
|- |
|||
| [[HDAC3]] || 9,000 || |
|||
|- |
|||
| [[HDAC4]] || 2,000,000 || Lower bound |
|||
|- |
|||
| [[HDAC5]] || 2,000,000 || Lower bound |
|||
|- |
|||
| [[HDAC6]] || 2,000,000 || Lower bound |
|||
|- |
|||
| [[HDAC7]] || 2,000,000 || Lower bound |
|||
|- |
|||
| [[HDAC8]] || 15,000 || |
|||
|- |
|||
| [[HDAC9]] || 2,000,000 || Lower bound |
|||
|- |
|||
| [[Carbonic anhydrase I|CA1]] || 511,000 || |
|||
|- |
|||
| [[Carbonic anhydrase I|CA2]] || 1,032,000 || |
|||
|- |
|||
! [[GPCR]] target !! [[pEC50|pEC<sub>50</sub>]] !! Entry note |
|||
|- |
|||
| [[FFAR2]] || 2.9–4.6 || Full agonist |
|||
|- |
|||
| [[FFAR3]] || 3.8–4.9 || Full agonist |
|||
|- |
|||
| [[NIACR1]] || <small>missing data</small> || Full agonist |
|||
|} |
|} |
||
[[File:Leloir pathway.png|540px|thumb|right|갈락토스 대사]] |
|||
포도당은 인체 내 대사의 주에너지원이다. 포도당은 갈락토스보다 안정하며, 적어도 하나의 당이 단백질 또는 지질에 부착된 분자인 비특이적 당포합체의 형성에 덜 민감하다. 이러한 이유로 많은 연구자들은 갈락토스에서 포도당으로의 신속한 전환을 위한 경로가 많은 생물종들에서 고도로 보존되어왔다고 추측하고 있다.<ref name=OMMBID72>{{cite web |url=http://www.ommbid.com/OMMBID/the_online_metabolic_and_molecular_bases_of_inherited_disease/b/abstract/part7/ch72 |title= Galactosemia |chapter=72 |vauthors=Fridovich-Keil JL, Walter JH |format= |work=The Online Metabolic and Molecular Bases of Inherited Disease |accessdate=}}<br />a 4 b 21 c 22 d 22</ref> |
|||
=== Pharmacodynamics === |
|||
갈락토스 대사의 주된 경로는 를루아르 경로이다. 그러나 인간과 몇몇 다른 생물종들에서는 몇 가지 대체 경로가 포함되어 있는 것으로 나타났다. 를루아르 경로는 β-D-갈락토스를 UDP-포도당으로 전환시키는 과정으로 구성된다. 첫 단계는 변광회전효소(mutarotase, GALM)에 의한 β-D-갈락토스의 α-D-갈락토스로의 전환이다. 를루아르 경로는 3가지 주요 효소를 통해 α-D-갈락토스를 UDP-포도당으로 전환시킨다. 갈락토카이네이스(GALK)는 α-D-갈락토스를 갈락토스-1-인산으로 인산화시킨다. 갈락토스-1-인산 유리딜 전이효소(GALT)는 UDP-포도당에서 UDP기를 갈락토스-1-인산으로 전이시켜 UDP-갈락토스를 형성한다. 마지막으로 UDP 갈락토스-4’-에피머화효소(GALE)는 UDP-갈락토스 및 UDP-포도당을 상호변환시켜 경로를 완성한다.<ref name=Bosch07>{{cite journal |author=Bosch AM |title=Classical galactosaemia revisited |journal=J. Inherit. Metab. Dis. |volume=29 |issue=4 |pages=516–25 |date=August 2006 |pmid=16838075 |doi=10.1007/s10545-006-0382-0 |url=}}<br /> a 517 b 516 c 519</ref> |
|||
Like other [[short-chain fatty acid]]s (SCFAs), butyrate is an agonist at the [[free fatty acid receptor]]s [[FFAR2]] and [[FFAR3]], which function as nutrient sensors which help regulate [[Energy balance (biology)|energy balance]];<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review">{{cite journal | vauthors = Bourassa MW, Alim I, Bultman SJ, Ratan RR | title = Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? | journal = Neurosci. Lett. | volume = 625 | issue = | pages = 56–63 | date = June 2016 | pmid = 26868600 | doi = 10.1016/j.neulet.2016.02.009 | url = http://www.sciencedirect.com/science/article/pii/S0304394016300775 | quote = Butyrate is an attractive therapeutic molecule because of its wide array of biological functions, such as its ability to serve as a histone deacetylase (HDAC) inhibitor, an energy metabolite to produce ATP and a G protein-coupled receptor (GPCR) activator. ... Histone acetylation is a post-translational modification by an epigenetic protein, which are proteins that bind to chromatin and influence chromatin structure to change the propensity that a gene is transcribed or repressed. Acetylated histones cause the chromatin structure to loosen by weakening electrostatic attraction between the histone proteins and the DNA backbone. This process enables transcription factors and the basal transcriptional machinery to bind and increases transcription. ... However, many studies have shown that at least some of these beneficial effects can be attributed NaB’s ability to increase acetylation around the promoters of neurotrophic factors, such as BDNF, GDNF and NGF and thus increasing their transcription [41], [42], [43], [44], [45], [46], [47] and [48]. ... Butyrate also signals through GPR109a ... Much of the butyrate produced in the colon is used as an energy source by the colonocytes, but some butyrate can also exit the colon through the portal vein, where the liver absorbs another large portion [74] and [75]. However, the distal colon is not connected to the portal vein, allowing for some systemic butyrate to be circulated. Indeed, there are many reports of high fiber diets increasing blood levels of circulating butyrate [75], [76] and [77]. These later reports raise the possibility that increases in circulating butyrate could affect CNS function directly.}}</ref><ref name="Review - General summary as of April 2015, excluding neuroepigenetic research" /><ref name="Review butyrate human T-cell HDACs" /> unlike the other SCFAs,<ref name="Review butyrate human T-cell HDACs" /> butyrate is also an agonist of [[niacin receptor 1]] (NIACR1, aka GPR109A).<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review" /><ref name="Review - General summary as of April 2015, excluding neuroepigenetic research" /><ref name="Review butyrate human T-cell HDACs" /> Butyric acid is utilized by mitochondria, particularly in colonocytes and by the liver, to generate [[adenosine triphosphate]] (ATP) during [[fatty acid metabolism]].<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review" /> Butyric acid is also an [[HDAC inhibitor]] (specifically, HDAC1, HDAC2, HDAC3, and HDAC8),<ref name="IUPHAR" /><ref name="BindingDB" /> a drug that inhibits the function of [[histone deacetylase]] enzymes, thereby favoring an acetylated state of [[histone]]s in cells.<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review" /> Histone acetylation loosens the structure of [[chromatin]] by reducing the [[electrostatic]] attraction between histones and [[DNA]].<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review" /> In general, it is thought that [[transcription factors]] will be unable to access regions where histones are tightly associated with DNA (i.e., non-acetylated, e.g., heterochromatin).{{mcn|date=October 2016}} Therefore, butyric acid is thought to enhance the transcriptional activity at promoters,<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review" /> which are typically silenced or downregulated due to histone deacetylase activity. |
|||
===Pharmacokinetics=== |
|||
Butyrate that is produced in the colon through microbial fermentation of dietary fiber is primarily absorbed and utilized by colonocytes and the liver{{#tag:ref|Most of the butyrate that is absorbed into [[blood plasma]] from the colon enters the circulatory system via the [[portal vein]];<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review" /> most of the butyrate that enters the circulatory system by this route is taken up by the liver.<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review" />|group="note"}} for the generation of ATP during energy metabolism;<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review" /> however, some butyrate is absorbed in the [[distal colon]], which is not connected to the portal vein, thereby allowing for the [[distribution (pharmacology)|systemic distribution]] of butyrate to multiple organ systems through the circulatory system.<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review" /> Butyrate that has reached systemic circulation can readily cross the [[blood-brain barrier]] via [[monocarboxylate transporter]]s (i.e., certain members of the [[Solute carrier family#Families|SLC16A group of transporters]]).<ref name="SCFA MCT-mediated BBB passage - 2005 review">{{cite journal | vauthors = Tsuji A | title = Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems | journal = NeuroRx | volume = 2 | issue = 1 | pages = 54–62 | year = 2005 | pmid = 15717057 | pmc = 539320 | doi = 10.1602/neurorx.2.1.54 | quote = Other in vivo studies in our laboratories indicated that several compounds including acetate, propionate, butyrate, benzoic acid, salicylic acid, nicotinic acid, and some β-lactam antibiotics may be transported by the MCT at the BBB.<sup>21</sup> ... Uptake of valproic acid was reduced in the presence of medium-chain fatty acids such as hexanoate, octanoate, and decanoate, but not propionate or butyrate, indicating that valproic acid is taken up into the brain via a transport system for medium-chain fatty acids, not short-chain fatty acids.}}</ref><ref name="SCFA MCT-mediated BBB passage - 2014 review">{{cite journal | vauthors = Vijay N, Morris ME | title = Role of monocarboxylate transporters in drug delivery to the brain | journal = Curr. Pharm. Des. | volume = 20 | issue = 10 | pages = 1487–98 | year = 2014 | pmid = 23789956 | pmc = 4084603 | doi = 10.2174/13816128113199990462| quote = Monocarboxylate transporters (MCTs) are known to mediate the transport of short chain monocarboxylates such as lactate, pyruvate and butyrate. ... MCT1 and MCT4 have also been associated with the transport of short chain fatty acids such as acetate and formate which are then metabolized in the astrocytes [78]. ... SLC5A8 is expressed in normal colon tissue, and it functions as a tumor suppressor in human colon with silencing of this gene occurring in colon carcinoma. This transporter is involved in the concentrative uptake of butyrate and pyruvate produced as a product of fermentation by colonic bacteria. }}</ref> Other transporters that mediate the passage of butyrate across lipid membranes include [[SLC5A8]] (SMCT1), [[SLC27A1]] (FATP1), and [[SLC27A4]] (FATP4).<ref name="IUPHAR" /><ref name="SCFA MCT-mediated BBB passage - 2014 review" /> |
|||
==== Metabolism {{anchor|Butanoate metabolism}} ==== |
|||
{{expand section|<ref name="Butyrate metabolism">{{cite web|title=Butanoate metabolism - Reference pathway|url=http://www.genome.jp/kegg-bin/show_pathway?map00650|website=Kyoto Encyclopedia of Genes and Genomes|publisher=Kanehisa Laboratories|date=1 November 2017|accessdate=1 February 2018}}</ref>|date=May 2015}} |
|||
Butyric acid is metabolized by various human [[XM-ligase]]s (ACSM1, ACSM2B, ASCM3, ACSM4, ACSM5, and ACSM6), also known as butyrate–CoA ligase.<ref name="HMDB">{{cite encyclopedia|title=Butyric acid|url=http://www.hmdb.ca/metabolites/HMDB00039|website=Human Metabolome Database|publisher=University of Alberta|accessdate=15 August 2015}}</ref> The metabolite produced by this reaction is [[butyryl–CoA]], and is produced as follows:<ref name="HMDB" /> |
|||
:Adenosine triphosphate + Butyric acid + Coenzyme A → Adenosine monophosphate + Pyrophosphate + Butyryl-CoA |
|||
As a [[short-chain fatty acid]], butyrate is utilized by [[Mitochondrion|mitochondria]] as an energy (i.e., [[adenosine triphosphate]] or ATP) source through [[fatty acid metabolism]]. |
|||
In humans, the butyrate prodrug [[tributyrin]] is metabolized by [[triacylglycerol lipase]] into [[dibutyrin]] and butyrate through the reaction:<ref name="BRENDA tributyrin">{{cite web| title=triacylglycerol lipase – Homo sapiens| url=http://www.brenda-enzymes.org/enzyme.php?ecno=3.1.1.3&Suchword=&organism%5B%5D=Homo+sapiens&show_tm=0| work=BRENDA| publisher=Technische Universität Braunschweig.| accessdate=25 May 2015}}</ref> |
|||
:Tributyrin + H20 = Dibutyrin + Butyrate |
|||
{{clear right}} |
|||
==Research== |
|||
===Peripheral therapeutic effects=== |
|||
Butyrate has numerous beneficial effects in humans on [[energy homeostasis]] and related diseases ([[diabetes]] and [[obesity]]), [[inflammation]], and [[immune function]] (e.g., it has pronounced [[antimicrobial]] and [[anticarcinogenic]] effects).<ref name="Review - General summary as of April 2015, excluding neuroepigenetic research">{{cite journal | vauthors = Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I | title = Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation | journal = Nutrients | volume = 7 | issue = 4 | pages = 2839–49 | year = 2015 | pmid = 25875123 | pmc = 4425176 | doi = 10.3390/nu7042839 | quote = Short-chain fatty acids (SCFAs) such as acetate, butyrate, and propionate, which are produced by gut microbial fermentation of dietary fiber, are recognized as essential host energy sources and act as signal transduction molecules via G-protein coupled receptors (FFAR2, FFAR3, OLFR78, GPR109A) and as epigenetic regulators of gene expression by the inhibition of histone deacetylase (HDAC). Recent evidence suggests that dietary fiber and the gut microbial-derived SCFAs exert multiple beneficial effects on the host energy metabolism not only by improving the intestinal environment, but also by directly affecting various host peripheral tissues.}}</ref><ref name="Review for diabetes" /> These effects occur through its utilization by mitochondria to generate {{abbr|ATP|adenosine triphosphate}} during [[fatty acid metabolism]] or through one or more of its [[histone-modifying enzyme]] targets (i.e., the [[Histone deacetylase#Classes of HDACs in higher eukaryotes|class I histone deacetylase]]s) and [[G-protein coupled receptor]] targets (i.e., [[FFAR2]], [[FFAR3]], and [[NIACR1]]).<ref name="Review - General summary as of April 2015, excluding neuroepigenetic research" /> |
|||
====Immunomodulation and inflammation==== |
|||
Butyrate's effects on the immune system are mediated through the inhibition of class I [[histone deacetylase]]s and activation of its [[G-protein coupled receptor]] targets: [[NIACR1]] (GPR109A), [[FFAR2]] (GPR43), and [[FFAR3]] (GPR41).<ref name="Review butyrate human T-cell HDACs" /><ref name="REVIEW butyrate human LL-37" /> Among the [[short-chain fatty acid]]s, butyrate is the most potent promoter of intestinal regulatory T cells ''[[in vitro]]'' and the only one among the group that is an [[NIACR1]] ligand.<ref name="Review butyrate human T-cell HDACs" /> It has been shown to be a critical mediator of the colonic inflammatory response. It possesses both preventive and therapeutic potential to counteract inflammation-mediated [[ulcerative colitis]] and [[colorectal cancer]]. |
|||
Butyrate has established antimicrobial properties in humans that are mediated through the [[antimicrobial peptide]] [[LL-37]], which it induces via [[HDAC]] inhibition on histone H3.<ref name="REVIEW butyrate human LL-37">{{cite journal | vauthors = Wang G | title = Human antimicrobial peptides and proteins | journal = Pharmaceuticals (Basel) | volume = 7 | issue = 5 | pages = 545–94 | year = 2014 | pmid = 24828484 | pmc = 4035769 | doi = 10.3390/ph7050545 | quote = The establishment of a link between light therapy, vitamin D and human cathelicidin LL-37 expression provides a completely different way for infection treatment. Instead of treating patients with traditional antibiotics, doctors may be able to use light or vitamin D [291,292]. Indeed using narrow-band UV B light, the level of vitamin D was increased in psoriasis patients (psoriasis is a common autoimmune disease on skin) [293]. In addition, other small molecules such as butyrate can induce LL-37 expression [294]. Components from Traditional Chinese Medicine may regulate the AMP expression as well [295]. These factors may induce the expression of a single peptide or multiple AMPs [296]. It is also possible that certain factors can work together to induce AMP expression. While cyclic AMP and butyrate synergistically stimulate the expression of chicken β-defensin 9 [297], 4-phenylbutyrate (PBA) and 1,25-dihydroxyvitamin D3 (or lactose) can induce AMP gene expression synergistically [294,298]. It appears that stimulation of LL-37 expression by histone deacetylase (HDAC) inhibitors is cell dependent. Trichostatin and sodium butyrate increased the peptide expression in human NCI-H292 airway epithelial cells but not in the primary cultures of normal nasal epithelial cells [299]. However, the induction of the human LL-37 expression may not be a general approach for bacterial clearance. During Salmonella enterica infection of human monocyte-derived macrophages, LL-37 is neither induced nor required for bacterial clearance [300].}}<br />[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4035769/table/pharmaceuticals-07-00545-t003/ Table 3: Select human antimicrobial peptides and their proposed targets]<br />[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4035769/table/pharmaceuticals-07-00545-t004/ Table 4: Some known factors that induce antimicrobial peptide expression]</ref><ref name="Primary - Butyrate and LL-37 target">{{cite journal | vauthors = Yonezawa H, Osaki T, Hanawa T, Kurata S, Zaman C, Woo TD, Takahashi M, Matsubara S, Kawakami H, Ochiai K, Kamiya S | title = Destructive effects of butyrate on the cell envelope of Helicobacter pylori | journal = J. Med. Microbiol. | volume = 61 | issue = Pt 4 | pages = 582–9 | year = 2012 | pmid = 22194341 | doi = 10.1099/jmm.0.039040-0 | url = }}</ref><ref name="Primary - LL-37 human pylori">{{cite journal | vauthors = McGee DJ, George AE, Trainor EA, Horton KE, Hildebrandt E, Testerman TL | title = Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL-37 | journal = Antimicrob. Agents Chemother. | volume = 55 | issue = 6 | pages = 2897–904 | year = 2011 | pmid = 21464244 | pmc = 3101455 | doi = 10.1128/AAC.00016-11 | url = }}</ref> Butyrate increases [[gene expression]] of [[FOXP3]] (the [[transcription factor|transcription regulator]] for {{abbr|Tregs|regulatory T cells}}) and promotes colonic [[regulatory T cell]]s (Tregs) through the inhibition of class I [[histone deacetylases]];<ref name="Review butyrate human T-cell HDACs" /><ref name="REVIEW butyrate human LL-37" /> through these actions, it increases the expression of [[interleukin 10]], an anti-inflammatory [[cytokine]].<ref name="REVIEW butyrate human LL-37" /><ref name="Review butyrate human T-cell HDACs">{{cite journal | vauthors = Hoeppli RE, Wu D, Cook L, Levings MK | title = The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome | journal = Front Immunol | volume = 6 | issue = | page = 61 | date = February 2015 | pmid = 25741338 | pmc = 4332351 | doi = 10.3389/fimmu.2015.00061 | quote = Specific species that have been recognized by their high levels of butyrate production include ''[[Faecalibacterium prausnitzii]]'' and the cluster IV and XIVa of genus ''Clostridium'' ... Administration of acetate, propionate, and butyrate in drinking water mimics the effect of ''Clostridium'' colonization in germ-free mice, resulting in an elevated Treg frequency in the colonic lamina propria and increased IL-10 production by these Tregs (180, 182). Of the three main SCFAs, butyrate has been found to be the most potent inducer of colonic Tregs. Mice fed a diet enriched in butyrylated starches have more colonic Tregs than those fed a diet containing propinylated or acetylated starches (181). Arpaia et al. tested an array of SCFAs purified from commensal bacteria and confirmed butyrate was the strongest SCFA-inducer of Tregs in vitro (180). Mechanistically, it has been proposed that butyrate, and possibly propionate, promote Tregs through inhibiting histone deacetylase (HDAC), causing increased acetylation of histone H3 in the Foxp3 CNS1 region, and thereby enhancing FOXP3 expression (180, 181). Short-chain fatty acids partially mediate their effects through G-protein coupled receptors (GPR), including GPR41, GPR43, and GPR109A. GPR41 and GPR43 are stimulated by all three major SCFAs (191), whereas GPR109A only interacts with butyrate (192).}}<br />[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4332351/figure/F1/ Figure 1: Microbial-derived molecules promote colonic Treg differentiation.]</ref> Butyrate also suppresses colonic inflammation by inhibiting the [[IFN-γ]]–[[STAT1]] signaling pathways, which is mediated partially through [[HDAC inhibitor|histone deacetylase inhibition]]. While transient IFN-γ signaling is generally associated with normal host [[immune response]], chronic IFN-γ signaling is often associated with chronic inflammation. It has been shown that butyrate inhibits activity of HDAC1 that is bound to the Fas gene promoter in T cells, resulting in hyperacetylation of the Fas promoter and up-regulation of [[Fas receptor]] on the T-cell surface.<ref name="pmid22517765">{{cite journal | vauthors = Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, Shi H, Robertson KD, Munn DH, Liu K | title = Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells | journal = Am. J. Physiol. Gastrointest. Liver Physiol. | volume = 302 | issue = 12 | pages = G1405–15 | year = 2012 | pmid = 22517765 | pmc = 3378095 | doi = 10.1152/ajpgi.00543.2011 | url = }}</ref> It is thus suggested that butyrate enhances apoptosis of T cells in the colonic tissue and thereby eliminates the source of inflammation (IFN-γ production).<ref name="pmid22517765" /> |
|||
Similar to other [[NIACR1]] agonists, butyrate also produces marked anti-inflammatory effects in a variety of tissues, including the brain, gastrointestinal tract, skin, and [[Inflammation#Vascular component|vascular tissue]].<ref name="Niacin neuroinflammation">{{cite journal | vauthors = Offermanns S, Schwaninger M | title = Nutritional or pharmacological activation of HCA(2) ameliorates neuroinflammation | journal = Trends Mol Med | volume = 21 | issue = 4 | pages = 245–255 | year = 2015 | pmid = 25766751 | doi = 10.1016/j.molmed.2015.02.002 | quote = Neuroinflammatory cells express HCA2, a receptor for the endogenous neuroprotective ketone body β-hydroxybutyrate (BHB) as well as for the drugs dimethyl fumarate (DMF) and nicotinic acid, which have established efficacy in the treatment of MS and experimental stroke, respectively. This review summarizes the evidence that HCA2 is involved in the therapeutic effects of DMF, nicotinic acid, and ketone bodies in reducing neuroinflammation.}}</ref><ref name="Niacin vascular inflammation">{{cite journal | vauthors = Chai JT, Digby JE, Choudhury RP | title = GPR109A and vascular inflammation | journal = Curr Atheroscler Rep | volume = 15 | issue = 5 | page = 325 | date = May 2013 | pmid = 23526298 | pmc = 3631117 | doi = 10.1007/s11883-013-0325-9 | quote = As GPR109A's primary pharmacological ligand in clinical use, niacin has been used for over 50 years in the treatment of cardiovascular disease, mainly due to its favourable effects on plasma lipoproteins. However, it has become apparent that niacin also possesses lipoprotein-independent effects that influence inflammatory pathways mediated through GPR109A.}}</ref><ref name="NIACR1 anti-inflammatory effects">{{cite journal | vauthors = Graff EC, Fang H, Wanders D, Judd RL | title = Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2 | journal = Metab. Clin. Exp. | volume = 65 | issue = 2 | pages = 102–113 | date= February 2016 | pmid = 26773933 | doi = 10.1016/j.metabol.2015.10.001 | quote = HCA2 is highly expressed on immune cells, including macrophages, monocytes, neutrophils and dermal dendritic cells, among other cell types. ... Recent studies demonstrate that HCA2 mediates profound anti-inflammatory effects in a variety of tissues, indicating that HCA2 may be an important therapeutic target for treating inflammatory disease processes.}}</ref><ref name="Niacin-NIACR1 in PD">{{cite journal | vauthors = Wakade C, Chong R | title = A novel treatment target for Parkinson's disease | journal = J. Neurol. Sci. | volume = 347 | issue = 1-2 | pages = 34–38 | date = December 2014 | pmid = 25455298 | doi = 10.1016/j.jns.2014.10.024 | quote = GPR109A and its agonists are known to exert anti-inflammatory actions in the skin, gut and retina.}}</ref> Butyrate binding at FFAR3 induces [[neuropeptide Y]] release and promotes the functional [[homeostasis]] of colonic mucosa and the enteric immune system.<ref name="Butyrate NPY review">{{cite journal | vauthors = Farzi A, Reichmann F, Holzer P | title = The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour | journal = Acta Physiol (Oxf) | volume = 213 | issue = 3 | pages = 603–27 | year = 2015 | pmid = 25545642 | pmc = 4353849 | doi = 10.1111/apha.12445 | quote = In the context of this review it is particularly worth noting that short chain fatty acids such as butyrate, which the colonic microbiota generates by fermentation of otherwise indigestible dietary fibre (Cherbut et al. 1998), stimulate L cells to release PYY via the G-protein coupled receptor Gpr41 (Samuel et al. 2008). In this way, short chain fatty acids can indirectly attenuate gastrointestinal motility as well as electrolyte and water secretion (Cox 2007b). More importantly, short chain fatty acids exert homeostatic actions on the function of the colonic mucosa and immune system (Hamer et al. 2008, Tazoe et al. 2008, Guilloteau et al. 2010, Macia et al. 2012a, Smith et al. 2013). Whether PYY plays a role in these effects of short chain fatty acids awaits to be investigated, but may be envisaged from the finding that PYY promotes mucosal cell differentiation (Hallden & Aponte 1997).}}</ref> |
|||
Butyric acid is important as an energy ([[adenosine triphosphate|ATP]]) source for cells lining the mammalian [[Large intestine|colon]] (colonocytes). Without butyric acid for energy, colon cells undergo upregulated autophagy (i.e., self-digestion).<ref>{{Cite journal|last=Donohoe|first=Dallas R.|last2=Garge|first2=Nikhil|last3=Zhang|first3=Xinxin|last4=Sun|first4=Wei|last5=O’Connell|first5=Thomas M.|last6=Bunger|first6=Maureen K.|last7=Bultman|first7=Scott J.|date=4 May 2011|title=The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon|journal=Cell Metabolism|volume=13|issue=5|pages=517–526|doi=10.1016/j.cmet.2011.02.018|issn=1550-4131|pmc=3099420|pmid=21531334}}</ref> |
|||
====Cancer==== |
|||
Butyrate produces different effects in healthy and cancerous cells; this is known as the "butyrate paradox". In particular, butyrate inhibits colonic tumor cells and promotes healthy colonic epithelial cells.<ref>{{cite journal|vauthors=Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K, Brummer RJ |title=Butyrate-induced transcriptional changes in human colonic mucosa |journal=PLOS ONE |volume=4 |issue=8 |pages=e6759 |year=2009 |pmid=19707587 |pmc=2727000 |doi=10.1371/journal.pone.0006759 |url=http://www.posone.org/article/info:doi%2F10.1371%2Fjournal.pone.0006759 |editor1-last=Bereswill |editor1-first=Stefan }}{{dead link|date=November 2016 |bot=InternetArchiveBot |fix-attempted=yes }}</ref> The signaling mechanism is not well understood.<ref>{{cite journal | vauthors = Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L | title = Oncogenic Ras promotes butyrate-induced apoptosis through inhibition of gelsolin expression | journal = The Journal of Biological Chemistry | volume = 279 | issue = 35 | pages = 36680–8 | date = August 2004 | pmid = 15213223 | doi = 10.1074/jbc.M405197200 | url = http://www.jbc.org/content/279/35/36680.full.pdf }}</ref> A review suggested that the chemopreventive benefits of butyrate depend in part on the amount, time of exposure with respect to the tumorigenic process, and type of fat in the diet.<ref name="lupton"/> The production of [[volatile fatty acids]] such as butyrate from fermentable fibers may contribute to the role of dietary fiber in colon cancer.<ref name="lupton"/> Short-chain fatty acids, which include butyric acid, are produced by beneficial [[Gut flora|colonic bacteria]] ([[probiotics]]) that feed on, or ferment prebiotics, which are plant products that contain adequate amounts of dietary fiber. These short-chain fatty acids benefit the colonocytes by increasing energy production and cell proliferation, and may protect against colon cancer.<ref>{{Cite book|title=Microbial Degradation Products Influence Colon Cancer Risk: the Butyrate Controversy|last=Lupton|first=Joanne R.|publisher=J. Nutr.|year=2004|isbn=|location=vol. 134 no. 2|pages=479–482}}</ref> |
|||
Conversely, some researchers have sought to eliminate butyrate and consider it a potential cancer driver.<ref>{{cite web|url=http://media.utoronto.ca/media-releases/low-carb-diet-cuts-risk-of-colon-cancer-study-finds/|title=Low-carb diet cuts risk of colon cancer, study finds {{!}} University of Toronto Media Room|website=media.utoronto.ca|access-date=2016-05-04}}</ref> Studies in mice indicate it drives transformation of [[MSH2|MSH2-deficient]] colon epithelial cells.<ref>{{Cite journal|last=Belcheva|first=Antoaneta|last2=Irrazabal|first2=Thergiory|last3=Robertson|first3=Susan J.|last4=Streutker|first4=Catherine|last5=Maughan|first5=Heather|last6=Rubino|first6=Stephen|last7=Moriyama|first7=Eduardo H.|last8=Copeland|first8=Julia K.|last9=Kumar|first9=Sachin|date=2014-07-17|title=Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells|journal=Cell|volume=158|issue=2|pages=288–299|doi=10.1016/j.cell.2014.04.051|issn=1097-4172|pmid=25036629}}</ref> It is important to note that these are related to a gene deficiency. Niacin, beta-hydroxybutyrate, and [[curcumin]] may be effective adjunct treatments if genetic issues are present.<ref>{{cite web | url=https://www.sciencedaily.com/releases/2009/04/090413141259.htm | title=Colon Cancer Shuts Down Receptor That Could Shut It Down | website=ScienceDaily | accessdate=2016-05-04}}</ref>{{ums|date=October 2016}} |
|||
====Diabetes==== |
|||
A review on the relationship between the microbiome and diabetes asserted that butyrate can induce "profound immunometabolic effects" in animal models and humans with [[type 2 diabetes]];<ref name="Review for diabetes">{{cite journal | vauthors = Tilg H, Moschen AR | title = Microbiota and diabetes: an evolving relationship | journal = Gut | volume = 63 | issue = 9 | pages = 1513–1521 | date = September 2014 | pmid = 24833634 | doi = 10.1136/gutjnl-2014-306928 | quote = Recent studies have suggested that gut bacteria play a fundamental role in diseases such as obesity, diabetes and cardiovascular disease. Data are accumulating in animal models and humans suggesting that obesity and type 2 diabetes (T2D) are associated with a profound dysbiosis. First human metagenome-wide association studies demonstrated highly significant correlations of specific intestinal bacteria, certain bacterial genes and respective metabolic pathways with T2D. Importantly, especially butyrate-producing bacteria such as Roseburia intestinalis and Faecalibacterium prausnitzii concentrations were lower in T2D subjects. This supports the increasing evidence, that butyrate and other short-chain fatty acids are able to exert profound immunometabolic effects.}}</ref> it also noted a relationship between the presence of obesity or diabetes and a state of marked [[dysbiosis]] in a host, which is not yet completely understood.<ref name="Review for diabetes" /> While acknowledging that there is strong evidence for the use of butyrate in such disorders, the review called for more research into the [[pathophysiology]] (i.e., biomolecular mechanisms) of these diseases, so as to improve therapeutic approaches to these diseases.<ref name="Review for diabetes" /> |
|||
===Neuroepigenetic effects=== |
|||
{{expand section|date=October 2016|<ref name="Butyrate pharmacodynamics and neuroepigenetic effects 2016 review" />}} |
|||
====Addiction==== |
|||
{{Psychostimulant addiction|align=right}} |
|||
The observation of a large number of [[Downregulation and upregulation|downregulated]] genes after [[methamphetamine]] withdrawal is consistent with previous results showing that methamphetamine can cause increased expression of histone deacetylases (HDACs) in the [[nucleus accumbens]] and the [[Striatum|dorsal striatum]]. Butyric acid is a HDAC inhibitor.<ref>{{Cite journal|last=Davie|first=James R.|date=2003-07-01|title=Inhibition of Histone Deacetylase Activity by Butyrate|url=http://jn.nutrition.org/content/133/7/2485S|journal=The Journal of Nutrition|language=en|volume=133|issue=7|pages=2485S–2493S|issn=0022-3166|pmid=12840228}}</ref> HDACs are enzymes that can cause histone deacetylation and repression of gene expression. HDACs are important regulators of synaptic formation, [[synaptic plasticity]], and [[long-term memory]] formation. Several HDACs also appear to play significant roles in various models of drug abuse and addiction.<ref>{{Cite journal|last=Cadet|first=Jean Lud|last2=Brannock|first2=Christie|last3=Jayanthi|first3=Subramaniam|last4=Krasnova|first4=Irina N.|date=1 January 2015|title=Transcriptional and Epigenetic Substrates of Methamphetamine Addiction and Withdrawal: Evidence from a Long-Access Self-Administration Model in the Rat|journal=Molecular Neurobiology|volume=51|issue=2|pages=696–717|doi=10.1007/s12035-014-8776-8|issn=0893-7648|pmc=4359351|pmid=24939695}}</ref> The local knockout of HDAC1, as well as chronic and continuous infusion of [[MS-275]], a pharmacological inhibitor highly selective in vitro for HDAC1, has been found with NAc suppressed cocaine-induced [[Stereotypy|locomotor]] sensitization in mice.<ref>{{Cite journal|last=Kennedy|first=Pamela J.|last2=Feng|first2=Jian|last3=Robison|first3=A.J.|last4=Maze|first4=Ian|last5=Badimon|first5=Ana|last6=Mouzon|first6=Ezekiell|last7=Chaudhury|first7=Dipesh|last8=Damez-Werno|first8=Diane M.|last9=Haggarty|first9=Stephen J.|date=1 April 2013|title=Class I HDAC Inhibition Blocks Cocaine-Induced Plasticity Through Targeted Changes in Histone Methylation|journal=Nature Neuroscience|volume=16|issue=4|pages=434–440|doi=10.1038/nn.3354|issn=1097-6256|pmc=3609040|pmid=23475113}}</ref> HDAC3 inhibitor RGFP966 has been shown to facilitate the extinction of cocaine-seeking behavior and prevent reinstatement of cocaine-conditioned place preference in mice.<ref>{{Cite journal|last=Malvaez|first=Melissa|last2=McQuown|first2=Susan C.|last3=Rogge|first3=George A.|last4=Astarabadi|first4=Mariam|last5=Jacques|first5=Vincent|last6=Carreiro|first6=Samantha|last7=Rusche|first7=James R.|last8=Wood|first8=Marcelo A.|date=12 February 2013|title=HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner|journal=Proceedings of the National Academy of Sciences of the United States of America|volume=110|issue=7|pages=2647–2652|doi=10.1073/pnas.1213364110|issn=0027-8424|pmc=3574934|pmid=23297220}}</ref> Histone deacetylase inhibitors have been shown to decrease cocaine, but not sucrose, self-administration in rats.<ref>{{Cite journal|last=Romieu|first=Pascal|last2=Host|first2=Lionel|last3=Gobaille|first3=Serge|last4=Sandner|first4=Guy|last5=Aunis|first5=Dominique|last6=Zwiller|first6=Jean|date=17 September 2008|title=Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats|journal=The Journal of Neuroscience|volume=28|issue=38|pages=9342–9348|doi=10.1523/JNEUROSCI.0379-08.2008|issn=1529-2401|pmid=18799668}}</ref> The beneficial bacteria that ferment probiotics and prebiotics to produce butyric acid have been shown to regulate behavior by means of the vagus nerve.<ref>{{Cite journal|last=Bravo|first=Javier A.|last2=Forsythe|first2=Paul|last3=Chew|first3=Marianne V.|last4=Escaravage|first4=Emily|last5=Savignac|first5=Hélène M.|last6=Dinan|first6=Timothy G.|last7=Bienenstock|first7=John|last8=Cryan|first8=John F.|date=2011-09-20|title=Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve|url=http://www.pnas.org/content/108/38/16050|journal=Proceedings of the National Academy of Sciences|language=en|volume=108|issue=38|pages=16050–16055|doi=10.1073/pnas.1102999108|issn=0027-8424|pmc=3179073|pmid=21876150}}</ref> |
|||
====Cognitive deficits and memory==== |
|||
갈락토스혈증(galactosemia)은 를루아르 경로의 효소들 중 하나에서 발생한 유전적 돌연변이로 인해 갈락토스를 적절하게 분해하지 못해서 생기는 선천적 대사이상증이다. 갈락토스혈증 환자는 소량의 갈락토스 섭취도 위험할 수 있다.<ref>{{cite web|url=https://www.ncbi.nlm.nih.gov/books/NBK1518|title=Classic Galactosemia and Clinical Variant Galactosemia|author=Gerard T Berry|work=nih.gov|accessdate=17 May 2015}}</ref> |
|||
Studies in rodents have found that the environment exerts an influence on epigenetic changes related to cognition, in terms of learning and memory;<ref>{{Cite book|url=https://www.amazon.com/The-Developing-Genome-Introduction-Epigenetics/dp/0199922349|title=The Developing Genome: An Introduction to Behavioral Epigenetics|date=2 March 2015|publisher=Oxford University Press|isbn=9780199922345|edition=1|language=English}}</ref> [[Environmental enrichment (neural)|environmental enrichment]] is correlated with increased [[histone acetylation]], and verification by administering [[histone deacetylase inhibitor]]s induced the sprouting of [[dendrite]]s, an increased number of [[synapse]]s, and reinstated learning behaviour and access to long-term memories.<ref>{{Cite journal|last=Fischer|first=Andre|last2=Sananbenesi|first2=Farahnaz|last3=Wang|first3=Xinyu|last4=Dobbin|first4=Matthew|last5=Tsai|first5=Li-Huei|date=10 May 2007|title=Recovery of learning and memory is associated with chromatin remodelling|journal=Nature|volume=447|issue=7141|pages=178–182|doi=10.1038/nature05772|issn=1476-4687|pmid=17468743}}</ref><ref name="Miller 24–27">{{Cite journal|last=Miller|first=Greg|date=2 July 2010|title=Epigenetics. The seductive allure of behavioral epigenetics|journal=Science|volume=329|issue=5987|pages=24–27|doi=10.1126/science.329.5987.24|issn=1095-9203|pmid=20595592}}</ref> Research has also linked learning and long-term memory formation to reversible epigenetic changes in the [[hippocampus]] and [[Cerebral cortex|cortex]] in animals with normal-functioning, undamaged brains.<ref name="Miller 24–27"/><ref>{{Cite journal|last=Gupta|first=Swati|last2=Kim|first2=Se Y.|last3=Artis|first3=Sonja|last4=Molfese|first4=David L.|last5=Schumacher|first5=Armin|last6=Sweatt|first6=J. David|last7=Paylor|first7=Richard E.|last8=Lubin|first8=Farah D.|date=10 March 2010|title=Histone methylation regulates memory formation|journal=The Journal of Neuroscience|volume=30|issue=10|pages=3589–3599|doi=10.1523/JNEUROSCI.3732-09.2010|issn=1529-2401|pmc=2859898|pmid=20219993}}</ref> In human studies, post-mortem brains from [[Alzheimer's disease|Alzheimer]]'s patients show increased histone de-acetylase levels.<ref>{{Cite journal|last=Peleg|first=Shahaf|last2=Sananbenesi|first2=Farahnaz|last3=Zovoilis|first3=Athanasios|last4=Burkhardt|first4=Susanne|last5=Bahari-Javan|first5=Sanaz|last6=Agis-Balboa|first6=Roberto Carlos|last7=Cota|first7=Perla|last8=Wittnam|first8=Jessica Lee|last9=Gogol-Doering|first9=Andreas|date=7 May 2010|title=Altered histone acetylation is associated with age-dependent memory impairment in mice|journal=Science|volume=328|issue=5979|pages=753–756|doi=10.1126/science.1186088|issn=1095-9203|pmid=20448184}}</ref><ref>{{Cite journal|last=Gräff|first=Johannes|last2=Rei|first2=Damien|last3=Guan|first3=Ji-Song|last4=Wang|first4=Wen-Yuan|last5=Seo|first5=Jinsoo|last6=Hennig|first6=Krista M.|last7=Nieland|first7=Thomas J. F.|last8=Fass|first8=Daniel M.|last9=Kao|first9=Patricia F.|date=8 March 2012|title=An epigenetic blockade of cognitive functions in the neurodegenerating brain|journal=Nature|volume=483|issue=7388|pages=222–226|doi=10.1038/nature10849|issn=1476-4687|pmc=3498952|pmid=22388814}}</ref> |
|||
== |
== See also == |
||
* [[:Category:Butyrates]] |
|||
갈락토스는 [[유제품]], [[아보카도]], [[사탕무]], 기타 [[고무]] 및 [[점액]]에서 발견된다. 또한 신체의 여러 [[조직 (생물학)|조직]]에서 [[당지질]]과 [[당단백질]]의 일부를 만드는 과정에서 합성된다. 또한 제 3세대 에탄올 생산 과정(대형 조류로부터)의 부산물이다. |
|||
* [[Histone]] |
|||
* [[Histone-modifying enzyme]] |
|||
** [[Histone acetylase]] |
|||
** [[Histone deacetylase]] |
|||
* [[Hydroxybutyric acid]]s |
|||
** [[α-Hydroxybutyric acid]] |
|||
** [[β-Hydroxybutyric acid]] |
|||
** [[γ-Hydroxybutyric acid]] |
|||
* [[β-Hydroxy β-methylbutyric acid]] |
|||
* [[β-Methylbutyric acid]] |
|||
* [[Synbiotics]] |
|||
{{clear}} |
|||
== |
== Notes == |
||
{{reflist|group=note}} |
|||
[[생쥐]], [[쥐]], [[초파리과|초파리]]를 D-갈락토스에 만성적으로 노출시킨 결과 [[노화]]가 촉진되었다.<ref>{{cite journal |vauthors=Pourmemar E, Majdi A, Haramshahi M, Talebi M, Karimi P, Sadigh-Eteghad S, |title=Intranasal Cerebrolysin Attenuates Learning and Memory Impairments in D-galactose-Induced Senescence in Mice |journal=Experimental Gerontology |volume=87 |pages=1622 |year=2017 |pmid=27894939|doi=10.1016/j.exger.2016.11.011}}</ref><ref>{{Cite journal | first1 = X. |
|||
| last2 = Zuo | first2 = P. |
|||
| last4 = Li |
|||
| last3 = Zhang |
|||
| last1 = Cui | first3 = Q. |
|||
| last6 = Long | first4 = X. |
|||
| last7 = Packer |
|||
| last8 = Liu |
|||
| last5 = Hu| first5 = Y. | first6 = J. | first7 = L. | first8 = J. |
|||
| title = Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: protective effects of R-alpha-lipoic acid |
|||
| journal = Journal of neuroscience research |
|||
| volume = 84 |
|||
| issue = 3 |
|||
| pages = 647654 |
|||
| year = 2006 |
|||
| pmid = 16710848 |
|||
| doi = 10.1002/jnr.20899 |
|||
}}</ref> 두 연구는 우유의 갈락토스와 [[난소암]] 사이의 연관성을 제시했다.<ref>{{cite journal |author=Cramer D |title=Lactase persistence and milk consumption as determinants of ovarian cancer risk |journal=Am J Epidemiol |volume=130 |issue=5 |pages=90410 |year=1989 |pmid=2510499}}</ref><ref>{{cite journal |vauthors=Cramer D, Harlow B, Willett W, Welch W, Bell D, Scully R, Ng W, Knapp R |title=Galactose consumption and metabolism in relation to the risk of ovarian cancer |journal=Lancet |volume=2 |issue=8654 |pages=6671 |year=1989 |pmid=2567871 |doi=10.1016/S0140-6736(89)90313-9}}</ref> 다른 연구에서는 갈락토스 대사 이상이 있더라도 갈락토스와 난소암 사이에는 상관관계가 없음을 보여주었다.<ref>{{cite journal |author1=Marc T. Goodman |author2=Anna H. Wu |author3=Ko-Hui Tung |author4=Katharine McDuffie |author5=Daniel W. Cramer |author6=Lynne R. Wilkens |author7=Keith Terada |author8=Juergen K. V. Reichardt |author9=Won G. Ng |title=Association of Galactose-1-Phosphate Uridyltransferase Activity and N314D Genotype with the Risk of Ovarian Cancer |journal=Am. J. Epidemiol. |volume=156 |issue=8 |pages=693701 |year=2002 |pmid=12370157 |doi=10.1093/aje/kwf104}}</ref><ref>{{cite journal |author1=Fung, W. L. Alan |author2=Risch, Harvey |author3=McLaughlin, John |author4=Rosen, Barry |author5=Cole, David |author6=Vesprini, Danny |author7=Narod, Steven A. |title=The N314D Polymorphism of Galactose-1-Phosphate Uridyl Transferase Does Not Modify the Risk of Ovarian Cancer |journal= Cancer Epidemiol Biomarkers Prev |volume=12 |issue=7 |pages=67880 |year=2003 |pmid=12869412}}</ref> 최근 하버드대 보건대학원에서 수행된 분석에 따르면 젖당 함유 식품과 난소암 사이에 특별한 상관관계를 보이지 않았고, 30 g/d의 젖당 소비에 대한 위험 증가치는 통계적으로 무의미했다.<ref>{{cite journal|author1=Genkinger, Jeanine M. |author2=Hunter, David J. |author3=Spiegelman, Donna |author4=Anderson, Kristin E. |author5=Arslan, Alan |author6=Beeson, W. Lawrence |author7=Buring, Julie E. |author8=Fraser, Gary E. |author9=Freudenheim, Jo L. |author10=Goldbohm, R. Alexandra |author11=Hankinson, Susan E. |author12=Jacobs, David R. Jr. |author13=Koushik, Anita |author14=Lacey, James V. Jr. |author15=Larsson, Susanna C. |author16=Leitzmann, Michael |author17=McCullough, Marji L. |author18=Miller, Anthony B. |author19=Rodriguez, Carmen |author20=Rohan, Thomas E. |author21=Schouten, Leo J. |author22=Shore, Roy |author23=Smit, Ellen |author24=Wolk, Alicja |author25=Zhang, Shumin M. |author26=Smith-Warner |author27=Stephanie A. |title=Dairy Products and Ovarian Cancer: A Pooled Analysis of 12 Cohort Studies|journal=Cancer Epidemiol Biomarkers Prev |volume=15 |pages=364372 |year=2006 |pmid=16492930 | doi = 10.1158/1055-9965.EPI-05-0484|issue=2}}</ref> 가능할 수 있는 위험을 확인하기 위해서는 더 많은 연구가 필요하다. |
|||
== References == |
|||
진행 중인 몇몇 연구들은 갈락토스가 국소 분절 사구체 경화증(focal segmental glomerulosclerosis)(신부전 및 단백뇨를 초래하는 신장병)의 치료에 역할을 할 수 있음을 시사한다.<ref>{{cite web|url=http://ndt.oxfordjournals.org/content/24/9/2938.full.pdf+html|title=FSGS permeability factor-associated nephrotic syndrome: remission after oral galactose therapy|work=oxfordjournals.org|accessdate=17 May 2015}}</ref> 이 효과는 FSGS 인자에 갈락토스가 결합된 결과일 수 있다.<ref>{{cite web|url=http://cjasn.asnjournals.org/content/5/11/2115.short|title=Circulating Permeability Factors in Idiopathic Nephrotic Syndrome and Focal Segmental Glomerulosclerosis|author=Ellen T. McCarthy|work=asnjournals.org|accessdate=17 May 2015}}</ref> |
|||
{{EB1911|wstitle=Butyric Acid}} |
|||
{{reflist|30em}} |
|||
{{reflist|group=Color legend}} |
|||
== External links == |
|||
갈락토스는 [[ABO식 혈액형]] 시스템 내에서 혈액형을 결정하는 적혈구에 존재하는 항원의 구성 요소이다. O항원 및 A항원에는 항원에 2개의 갈락토스 [[단위체]]가 있고, B항원에는 3개의 갈락토스 단위체가 있다.<ref name="Raven and Johnson">{{cite book|title=Understanding Biology|edition=3rd|author1=Peter H. Raven |author2=George B. Johnson |pages=203|isbn=0-697-22213-6|year=1995|editor=Carol J. Mills|publisher=WM C. Brown}}</ref> |
|||
{{Commons category|Butyric acid}} |
|||
*[https://www.cdc.gov/niosh/ipcsneng/neng1334.html International Chemical Safety Card 1334] |
|||
*[http://jn.nutrition.org/cgi/content/full/134/2/479 2004 review of the scientific evidence on butanoate/butyrate vs. colon cancer] |
|||
{{Fatty acids}} |
|||
2개의 갈락토스로 구성된 이당류인 갈락토스-α-1,3-갈락토스(α-gal)는 포유류 고기에 존재하는 잠재적인 [[알레르기 항원]]으로 인식되어 왔다. 론스타 진드기(lone star tick)에 물리면 α-gal 알레르기가 유발될 수 있다. |
|||
{{HDAC inhibitors}} |
|||
{{GABA metabolism and transport modulators}} |
|||
{{Authority control}} |
|||
{{DEFAULTSORT:Butyric Acid}} |
|||
== 역사 == |
|||
[[Category:GABA analogues]] |
|||
1855년에 에르트만(E. O. Erdmann)은 젖당의 가수분해가 포도당 이외의 물질을 생성한다고 언급하였다.<ref>See: |
|||
[[Category:Flavors]] |
|||
* Eduard Otto Erdmann (1855) Dissertation: ''Dissertatio de saccharo lactico et amylaceo'' [Dissertation on milk sugar and starch](University of Berlin). |
|||
[[Category:Alkanoic acids]] |
|||
* ''Jahresbericht über die Fortschritte der reinen, pharmaceutischen und technischen Chemie'', … [Annual report on progress in pure, pharmaceutical, and technical chemistry, … ] (1855), [https://books.google.com/books?id=7LVZAAAAcAAJ&pg=PA671#v=onepage&q&f=false pages 671–673;] see especially p. 673.</ref> 갈락토스는 1856년 [[루이 파스퇴르]]에 의해 처음으로 분리, 연구되었고,<ref>Pasteur (1856) [https://archive.org/stream/ComptesRendusAcademieDesSciences0042/ComptesRendusAcadmieDesSciences-Tome042-Janvier-juin1856#page/n350/mode/1up "Note sur le sucre de lait"] (Note on milk sugar), ''Comptes rendus'', '''42''' : 347–351.</ref> 파스퇴르는 갈락토스를 "젖당(lactose)"이라고 불렀다.<ref>Pasteur (1856), p. 348. From page 348: ''"Je propose de le nommer ''lactose''."'' (I propose to name it ''lactose''.)</ref> 1860년에 베르틀로(Berthelot)는 갈락토스를 "갈락토스(galactose)" 또는 "글루코스 락티크(glucose lactique)로 개명하였다.<ref>Marcellin Berthelot, ''Chimie organique fondée sur la synthèse'' [Organic chemistry based on synthesis] (Paris, France: Mallet-Bachelier, 1860), vol. 2, [https://books.google.com/books?id=7AtQYV5FlVwC&pg=PA248#v=onepage&q&f=false pp. 248–249].</ref><ref>"Galactose" — from the [[Ancient Greek]] [[wikt:γάλακτος|γάλακτος]] (gálaktos, “milk”).</ref> 1894년에 에밀 피셔(Emil Fischer)와 로버트 모렐(Robert Morrell)은 갈락토스의 입체 배치(configuration)를 밝혀냈다.<ref>Emil Fischer and Robert S. Morrell (1894) [http://gallica.bnf.fr/ark:/12148/bpt6k90732c/f403.image.langEN "Ueber die Configuration der Rhamnose und Galactose"] (On the configuration of rhamnose and galactose), ''Berichte der Deutschen chemischen Gesellschaft zu Berlin'', '''27''' : 382–394. The configuration of galactose appears on page 385.</ref> |
|||
[[Category:Fatty acids]] |
|||
[[Category:Butyrates]] |
|||
[[Category:Foul-smelling chemicals]] |
|||
[[Category:Biomolecules]] |
|||
[[Category:Histone deacetylase inhibitors]] |
|||
==각주== |
==각주== |
||
{{각주}} |
{{각주}} |
||
{{ |
{{지방산}} |
||
[[분류: |
[[분류:지방산]] |
||
[[분류:영양]] |
|||
[[분류:감미료]] |
|||
2018년 2월 26일 (월) 17:02 판
| |

| |
| 일반적인 성질 | |
|---|---|
| IUPAC 이름 | Butanoic acid |
| 화학식 | C4H8O2 |
| CAS 번호 | 107-92-6 |
| 물리적 성질 | |
| 분자량 | 88.11 g/mol |
| 녹는점 | 268.05 K -5.1 °C 22.82 °F |
| 끓는점 | 436.9 K 163.75 °C 326.75 °F |
| 밀도 | 0.9528 g/cm3 |
| 형태 | 무색 유성 액체 |
| 열화학적 성질 | |
| 안전성 | |
뷰티르산(영어: butyric acid) (그리스어 "βούτῡρον"에서 유래, "butter"를 의미함)은 계통명은 부탄산(butanoic acid)이고, BTA로 약칭되며,[1] 화학식이 CH3CH2CH2-COOH 인 카복실산이다. 뷰티르산의 염 및 에스터(에스테르)는 뷰티레이트(butyrate) 또는 부타노에이트(butanoate)로 알려져 있다. 뷰티르산은 우유 특히 염소, 양 및 아메리카 들소의 젖, 버터, 파마산 치즈 및 혐기성 발효 산물(결장 및 액취증 포함)에서 발견된다. 또한 뷰티르산은 허쉬 공정에 의해 생산된 밀크 초콜릿에서 발견되거나, 허쉬 초콜릿의 맛을 모방하기 위해 첨가된 것으로 추측된다.[2] 뷰티르산은 사람의 구토물에 존재하며, 불쾌한 냄새를 낸다.[3] 뷰티르산은 불쾌한 냄새와 콕 쏘는 맛을 가지며, 에테르와 유사한 약간 감미로운 뒷맛을 가진다. 개와 같은 좋은 냄새 탐지 능력이 있는 포유류는 뷰티르산을 10ppb(parts per billion)로 탐지할 수 있는 반면에 사람은 10ppm(parts per million)이상의 농도에서 뷰티르산을 탐지할 수 있다.
뷰티르산은 1814년 프랑스의 화학자 미셀 외젠 슈프뢰이(Michel Eugène Chevreul)에 의해 불순한 형태로 처음 관찰되었다. 1818년까지 슈브외이는 뷰티르산을 특징짓기 위해 충분하게 정제했다. 그러나 슈브뢰이는 뷰티르산에 대한 초기 연구를 발표하지 않았고, 대신 프랑스 파리에 있는 과학 아카데미에 원고 형태로 연구 결과물을 맡겼다. 또한 프랑스의 화학자인 앙리 브라코노(Henri Braconnot)도 버터의 성분을 연구하고 연구 결과를 발표했는데 이것이 우선권에 대한 논쟁으로 이어졌다. 1815년 초에 슈브뢰이는 버터 냄새의 원인이 되는 물질을 발견했다고 주장했다.[4] 1817년에 슈브뢰이는 뷰티르산의 성질에 관한 연구 결과를 발표하고, 뷰티르산의 이름을 지었다.[5] 그러나, 1823년에 이르러서야 뷰티르산의 특성이 자세히 밝혀졌다.[6] 뷰티르산이란 이름은 처음 발견된 물질인 butyrum (또는 buturum)이라는 버터의 라틴어 단어에서 유래되었다.
화학
뷰티르산은 동물성 지방에서 에스터의 형태로 발견되는 지방산이다. 뷰티르산의 트라이글리세라이드는 버터의 3~4%를 차지한다. 버터가 산패할 때, 가수분해에 의해 글리세라이드로부터 뷰티르산이 유리되어 불쾌한 냄새가 난다. 뷰티르산은 짧은 사슬 지방산이라고 불리는 지방산 하위 그룹의 주요 구성원이다. 뷰티르산은 염기와 강력한 산화제와 반응하여 많은 금속을 공격하는 중-강산이다.[7]
뷰티르산은 물, 에탄올 및 에테르에 쉽게 용해되는 유성의 무색 액체이며 염화 칼슘과 같은 염으로 포화되어 액상으로부터 분리될 수 있다. It is oxidized to carbon dioxide and acetic acid using potassium dichromate and sulfuric acid, while alkaline potassium permanganate oxidizes it to carbon dioxide. The calcium salt, Ca(C4H7O2)2·H2O, is less soluble in hot water than in cold.
Butyric acid has a structural isomer called isobutyric acid (2-methylpropanoic acid).
Safety
Personal protective equipment such as rubber or PVC gloves, protective eye goggles, and chemical-resistant clothing and shoes are used to minimize risks when handling butyric acid.
Inhalation of butyric acid may result in soreness of throat, coughing, a burning sensation, and laboured breathing. Ingestion of the acid may result in abdominal pain, shock, and collapse. Physical exposure to the acid may result in pain, blistering and skin burns, while exposure to the eyes may result in pain, severe deep burns and loss of vision.[7]
Production
It is industrially prepared by the fermentation of sugar or starch, brought about by the addition of putrefying cheese, with calcium carbonate added to neutralize the acids formed in the process. The butyric fermentation of starch is aided by the direct addition of Bacillus subtilis. Salts and esters of the acid are called butyrates or butanoates.
Butyric acid or fermentation butyric acid is also found as a hexyl ester hexyl butyrate in the oil of Heracleum giganteum (a type of hogweed) and as the octyl ester octyl butyrate in parsnip (Pastinaca sativa); it has also been noticed in skin flora and perspiration.
Uses
Butyric acid is used in the preparation of various butyrate esters. Low-molecular-weight esters of butyric acid, such as methyl butyrate, have mostly pleasant aromas or tastes. As a consequence, they are used as food and perfume additives. It is also used as an animal feed supplement due to the ability to reduce pathogenic bacterial colonization.[8] It is an approved food flavoring in the EU FLAVIS database (number 08.005).
Due to its powerful odor, it has also been used as a fishing bait additive.[9] Many of the commercially available flavors used in carp (Cyprinus carpio) baits use butyric acid as their ester base; however, it is not clear whether fish are attracted by the butyric acid itself or the substances added to it. Butyric acid was, however, one of the few organic acids shown to be palatable for both tench and bitterling.[10]
The substance has also been used as a stink bomb by Sea Shepherd Conservation Society to disrupt Japanese whaling crews,[11] as well as by anti-abortion protesters to disrupt abortion clinics.[12]
Butyric acid, along with acetic acid, can be reacted with cellulose to produce the organic ester cellulose acetate butyrate (CAB), which is used in a wide variety of tools, parts, and coatings and is more resistant to degradation than cellulose acetate.[13] However, CAB can degrade with exposure to heat and moisture, releasing butyric acid.[14] This process is sometimes observed in the unpleasant, vomit-like odor of aging screwdrivers and other hand tools.[15]
Biochemistry
Microbial biosynthesis
틀:Missing information Butyrate is produced as end-product of a fermentation process solely performed by obligate anaerobic bacteria. Fermented Kombucha "tea" includes butyric acid as a result of the fermentation. This fermentation pathway was discovered by Louis Pasteur in 1861. Examples of butyrate-producing species of bacteria:
- Clostridium butyricum
- Clostridium kluyveri
- Clostridium pasteurianum
- Faecalibacterium prausnitzii
- Fusobacterium nucleatum
- Butyrivibrio fibrisolvens
- Eubacterium limosum
The pathway starts with the glycolytic cleavage of glucose to two molecules of pyruvate, as happens in most organisms. Pyruvate is then oxidized into acetyl coenzyme A using a unique mechanism that involves an enzyme system called pyruvate:ferredoxin oxidoreductase. Two molecules of carbon dioxide (CO2) and two molecules of elemental hydrogen (H2) are formed as waste products from the cell. Then,
| Action | Responsible enzyme |
|---|---|
| Acetyl coenzyme A converts into acetoacetyl coenzyme A | acetyl-CoA-acetyl transferase |
| Acetoacetyl coenzyme A converts into β-hydroxybutyryl CoA | β-hydroxybutyryl-CoA dehydrogenase |
| β-hydroxybutyryl CoA converts into crotonyl CoA | crotonase |
| Crotonyl CoA converts into butyryl CoA (CH3CH2CH2C=O-CoA) | butyryl CoA dehydrogenase |
| A phosphate group replaces CoA to form butyryl phosphate | phosphobutyrylase |
| The phosphate group joins ADP to form ATP and butyrate | butyrate kinase |
ATP is produced, as can be seen, in the last step of the fermentation. Three molecules of ATP are produced for each glucose molecule, a relatively high yield. The balanced equation for this fermentation is
- C6H12O6 → C4H8O2 + 2 CO2 + 2 H2
Several species form acetone and n-butanol in an alternative pathway, which starts as butyrate fermentation. Some of these species are:
- Clostridium acetobutylicum, the most prominent acetone and propianol producer, used also in industry
- Clostridium beijerinckii
- Clostridium tetanomorphum
- Clostridium aurantibutyricum
These bacteria begin with butyrate fermentation, as described above, but, when the pH drops below 5, they switch into butanol and acetone production to prevent further lowering of the pH. Two molecules of butanol are formed for each molecule of acetone.
The change in the pathway occurs after acetoacetyl CoA formation. This intermediate then takes two possible pathways:
- acetoacetyl CoA → acetoacetate → acetone
- acetoacetyl CoA → butyryl CoA → butyraldehyde → butanol
Highly-fermentable fiber residues, such as those from resistant starch, oat bran, pectin, and guar are transformed by colonic bacteria into short-chain fatty acids (SCFA) including butyrate, producing more SCFA than less fermentable fibers such as celluloses.[16] One study found that resistant starch consistently produces more butyrate than other types of dietary fiber.[17] The production of SCFA from fibers in ruminant animals such as cattle is responsible for the butyrate content of milk and butter.[18]
Fructans are another source of prebiotic soluble dietary fibers. They are often found in the soluble fibers of foods which are high in sulfur, such as the Allium and Cruciferous vegetables. Sources of fructans include wheat (although some wheat strains such as spelt contain lower amounts),[19] rye, barley, onion, garlic, Jerusalem and globe artichoke, asparagus, beetroot, chicory, dandelion leaves, leek, radicchio, the white part of spring onion, broccoli, brussels sprouts, cabbage, fennel and prebiotics such as fructooligosaccharides (FOS), oligofructose and inulin.[20][21] While many of these foods lack in butyrate production compared to resistant starch, they do have a number of benefits. They generally possess a low glycemic index which appeals well to diabetics. They also appeal to those on the ketogenic diet who benefit from beta-hydroxybutyric acid, which is a HDAC inhibitor which can cross the blood brain barrier and be used as fuel in the mitochondria of brain cells.[22] Other HDAC inhibitors in these butyrate producing foods are sulforaphane,[23] which has promise in inhibiting human breast cancer cells.[24] Sulforaphane has also been shown to promote hair growth in mice,[25] it contains compounds which prevent ulcers,[26] and helps with cognitive function in rats.[27] It is good to note that sulforaphane in broccoli is destroyed if prepared improperly.[28] Diallyl disulfide found in the fructans containing garlic has been shown to reduce chemical toxicity and carcinogenesis in rodents,[29] and shows synergestic benefits with butyrate when it comes to inhibiting the growth of human cancer tumor cells in the colon.[30]
Pharmacology
| Inhibited enzyme | IC50 (nM) | Entry note |
|---|---|---|
| HDAC1 | 16,000 | |
| HDAC2 | 12,000 | |
| HDAC3 | 9,000 | |
| HDAC4 | 2,000,000 | Lower bound |
| HDAC5 | 2,000,000 | Lower bound |
| HDAC6 | 2,000,000 | Lower bound |
| HDAC7 | 2,000,000 | Lower bound |
| HDAC8 | 15,000 | |
| HDAC9 | 2,000,000 | Lower bound |
| CA1 | 511,000 | |
| CA2 | 1,032,000 | |
| GPCR target | pEC50 | Entry note |
| FFAR2 | 2.9–4.6 | Full agonist |
| FFAR3 | 3.8–4.9 | Full agonist |
| NIACR1 | missing data | Full agonist |
Pharmacodynamics
Like other short-chain fatty acids (SCFAs), butyrate is an agonist at the free fatty acid receptors FFAR2 and FFAR3, which function as nutrient sensors which help regulate energy balance;[33][34][35] unlike the other SCFAs,[35] butyrate is also an agonist of niacin receptor 1 (NIACR1, aka GPR109A).[33][34][35] Butyric acid is utilized by mitochondria, particularly in colonocytes and by the liver, to generate adenosine triphosphate (ATP) during fatty acid metabolism.[33] Butyric acid is also an HDAC inhibitor (specifically, HDAC1, HDAC2, HDAC3, and HDAC8),[31][32] a drug that inhibits the function of histone deacetylase enzymes, thereby favoring an acetylated state of histones in cells.[33] Histone acetylation loosens the structure of chromatin by reducing the electrostatic attraction between histones and DNA.[33] In general, it is thought that transcription factors will be unable to access regions where histones are tightly associated with DNA (i.e., non-acetylated, e.g., heterochromatin).틀:Mcn Therefore, butyric acid is thought to enhance the transcriptional activity at promoters,[33] which are typically silenced or downregulated due to histone deacetylase activity.
Pharmacokinetics
Butyrate that is produced in the colon through microbial fermentation of dietary fiber is primarily absorbed and utilized by colonocytes and the liver[note 1] for the generation of ATP during energy metabolism;[33] however, some butyrate is absorbed in the distal colon, which is not connected to the portal vein, thereby allowing for the systemic distribution of butyrate to multiple organ systems through the circulatory system.[33] Butyrate that has reached systemic circulation can readily cross the blood-brain barrier via monocarboxylate transporters (i.e., certain members of the SLC16A group of transporters).[36][37] Other transporters that mediate the passage of butyrate across lipid membranes include SLC5A8 (SMCT1), SLC27A1 (FATP1), and SLC27A4 (FATP4).[31][37]
Metabolism
이 문단은 아직 미완성입니다. 여러분의 지식으로 알차게 문서를 완성해 갑시다. |
Butyric acid is metabolized by various human XM-ligases (ACSM1, ACSM2B, ASCM3, ACSM4, ACSM5, and ACSM6), also known as butyrate–CoA ligase.[3] The metabolite produced by this reaction is butyryl–CoA, and is produced as follows:[3]
- Adenosine triphosphate + Butyric acid + Coenzyme A → Adenosine monophosphate + Pyrophosphate + Butyryl-CoA
As a short-chain fatty acid, butyrate is utilized by mitochondria as an energy (i.e., adenosine triphosphate or ATP) source through fatty acid metabolism.
In humans, the butyrate prodrug tributyrin is metabolized by triacylglycerol lipase into dibutyrin and butyrate through the reaction:[38]
- Tributyrin + H20 = Dibutyrin + Butyrate
Research
Peripheral therapeutic effects
Butyrate has numerous beneficial effects in humans on energy homeostasis and related diseases (diabetes and obesity), inflammation, and immune function (e.g., it has pronounced antimicrobial and anticarcinogenic effects).[34][39] These effects occur through its utilization by mitochondria to generate ATP during fatty acid metabolism or through one or more of its histone-modifying enzyme targets (i.e., the class I histone deacetylases) and G-protein coupled receptor targets (i.e., FFAR2, FFAR3, and NIACR1).[34]
Immunomodulation and inflammation
Butyrate's effects on the immune system are mediated through the inhibition of class I histone deacetylases and activation of its G-protein coupled receptor targets: NIACR1 (GPR109A), FFAR2 (GPR43), and FFAR3 (GPR41).[35][40] Among the short-chain fatty acids, butyrate is the most potent promoter of intestinal regulatory T cells in vitro and the only one among the group that is an NIACR1 ligand.[35] It has been shown to be a critical mediator of the colonic inflammatory response. It possesses both preventive and therapeutic potential to counteract inflammation-mediated ulcerative colitis and colorectal cancer.
Butyrate has established antimicrobial properties in humans that are mediated through the antimicrobial peptide LL-37, which it induces via HDAC inhibition on histone H3.[40][41][42] Butyrate increases gene expression of FOXP3 (the transcription regulator for Tregs) and promotes colonic regulatory T cells (Tregs) through the inhibition of class I histone deacetylases;[35][40] through these actions, it increases the expression of interleukin 10, an anti-inflammatory cytokine.[40][35] Butyrate also suppresses colonic inflammation by inhibiting the IFN-γ–STAT1 signaling pathways, which is mediated partially through histone deacetylase inhibition. While transient IFN-γ signaling is generally associated with normal host immune response, chronic IFN-γ signaling is often associated with chronic inflammation. It has been shown that butyrate inhibits activity of HDAC1 that is bound to the Fas gene promoter in T cells, resulting in hyperacetylation of the Fas promoter and up-regulation of Fas receptor on the T-cell surface.[43] It is thus suggested that butyrate enhances apoptosis of T cells in the colonic tissue and thereby eliminates the source of inflammation (IFN-γ production).[43]
Similar to other NIACR1 agonists, butyrate also produces marked anti-inflammatory effects in a variety of tissues, including the brain, gastrointestinal tract, skin, and vascular tissue.[44][45][46][47] Butyrate binding at FFAR3 induces neuropeptide Y release and promotes the functional homeostasis of colonic mucosa and the enteric immune system.[48]
Butyric acid is important as an energy (ATP) source for cells lining the mammalian colon (colonocytes). Without butyric acid for energy, colon cells undergo upregulated autophagy (i.e., self-digestion).[49]
Cancer
Butyrate produces different effects in healthy and cancerous cells; this is known as the "butyrate paradox". In particular, butyrate inhibits colonic tumor cells and promotes healthy colonic epithelial cells.[50] The signaling mechanism is not well understood.[51] A review suggested that the chemopreventive benefits of butyrate depend in part on the amount, time of exposure with respect to the tumorigenic process, and type of fat in the diet.[16] The production of volatile fatty acids such as butyrate from fermentable fibers may contribute to the role of dietary fiber in colon cancer.[16] Short-chain fatty acids, which include butyric acid, are produced by beneficial colonic bacteria (probiotics) that feed on, or ferment prebiotics, which are plant products that contain adequate amounts of dietary fiber. These short-chain fatty acids benefit the colonocytes by increasing energy production and cell proliferation, and may protect against colon cancer.[52]
Conversely, some researchers have sought to eliminate butyrate and consider it a potential cancer driver.[53] Studies in mice indicate it drives transformation of MSH2-deficient colon epithelial cells.[54] It is important to note that these are related to a gene deficiency. Niacin, beta-hydroxybutyrate, and curcumin may be effective adjunct treatments if genetic issues are present.[55]틀:Ums
Diabetes
A review on the relationship between the microbiome and diabetes asserted that butyrate can induce "profound immunometabolic effects" in animal models and humans with type 2 diabetes;[39] it also noted a relationship between the presence of obesity or diabetes and a state of marked dysbiosis in a host, which is not yet completely understood.[39] While acknowledging that there is strong evidence for the use of butyrate in such disorders, the review called for more research into the pathophysiology (i.e., biomolecular mechanisms) of these diseases, so as to improve therapeutic approaches to these diseases.[39]
Neuroepigenetic effects
이 문단은 아직 미완성입니다. 여러분의 지식으로 알차게 문서를 완성해 갑시다. |
Addiction
틀:Psychostimulant addiction The observation of a large number of downregulated genes after methamphetamine withdrawal is consistent with previous results showing that methamphetamine can cause increased expression of histone deacetylases (HDACs) in the nucleus accumbens and the dorsal striatum. Butyric acid is a HDAC inhibitor.[56] HDACs are enzymes that can cause histone deacetylation and repression of gene expression. HDACs are important regulators of synaptic formation, synaptic plasticity, and long-term memory formation. Several HDACs also appear to play significant roles in various models of drug abuse and addiction.[57] The local knockout of HDAC1, as well as chronic and continuous infusion of MS-275, a pharmacological inhibitor highly selective in vitro for HDAC1, has been found with NAc suppressed cocaine-induced locomotor sensitization in mice.[58] HDAC3 inhibitor RGFP966 has been shown to facilitate the extinction of cocaine-seeking behavior and prevent reinstatement of cocaine-conditioned place preference in mice.[59] Histone deacetylase inhibitors have been shown to decrease cocaine, but not sucrose, self-administration in rats.[60] The beneficial bacteria that ferment probiotics and prebiotics to produce butyric acid have been shown to regulate behavior by means of the vagus nerve.[61]
Cognitive deficits and memory
Studies in rodents have found that the environment exerts an influence on epigenetic changes related to cognition, in terms of learning and memory;[62] environmental enrichment is correlated with increased histone acetylation, and verification by administering histone deacetylase inhibitors induced the sprouting of dendrites, an increased number of synapses, and reinstated learning behaviour and access to long-term memories.[63][64] Research has also linked learning and long-term memory formation to reversible epigenetic changes in the hippocampus and cortex in animals with normal-functioning, undamaged brains.[64][65] In human studies, post-mortem brains from Alzheimer's patients show increased histone de-acetylase levels.[66][67]
See also
- Category:Butyrates
- Histone
- Histone-modifying enzyme
- Hydroxybutyric acids
- β-Hydroxy β-methylbutyric acid
- β-Methylbutyric acid
- Synbiotics
Notes
- ↑ Most of the butyrate that is absorbed into blood plasma from the colon enters the circulatory system via the portal vein;[33] most of the butyrate that enters the circulatory system by this route is taken up by the liver.[33]
References
![]() 본 문서에는 현재 퍼블릭 도메인에 속한 브리태니커 백과사전 제11판의 내용을 기초로 작성된 내용이 포함되어 있습니다.
본 문서에는 현재 퍼블릭 도메인에 속한 브리태니커 백과사전 제11판의 내용을 기초로 작성된 내용이 포함되어 있습니다.
- ↑ “Butanoic Acid”. ALS Environmental. 2014년 6월 13일에 확인함.
- ↑ Moskin, Julia (2008년 2월 13일). “Dark may be king, but milk chocolate makes a move”. 《The New York Times》. 2016년 1월 1일에 확인함.
- ↑ 가 나 다 〈Butyric acid〉. 《Human Metabolome Database》. University of Alberta. 2015년 8월 15일에 확인함.
- ↑ Chevreul (1815) "Lettre de M. Chevreul à MM. les rédacteurs des Annales de chimie" (Letter from Mr. Chevreul to the editors of the Annals of Chemistry), Annales de chimie, 94 : 73–79; in a footnote spanning pages 75–76, he mentions that he had found a substance that is responsible for the smell of butter.
- ↑ Chevreul (1817) "Extrait d'une lettre de M. Chevreul à MM. les Rédacteurs du Journal de Pharmacie" (Extract of a letter from Mr. Chevreul to the editors of the Journal of Pharmacy), Journal de Pharmacie et des sciences accessoires, 3 : 79–81. On p. 81, he named butyric acid: "Ce principe, que j'ai appelé depuis acid butérique, … " (This principle [i.e., constituent], which I have since named "butyric acid", … )
- ↑ E. Chevreul, Recherches chimiques sur les corps gras d'origine animale [Chemical researches on fatty substances of animal origin] (Paris, France: F.G. Levrault, 1823), pages 115–133.
- ↑ 가 나 ICSC 1334 – BUTYRIC ACID. Inchem.org (23 November 1998). Retrieved on 2014-03-31.
- ↑ Supplementation of Coated Butyric Acid in the Feed Reduces Colonization and Shedding of Salmonella in Poultry. Ps.fass.org. Retrieved on 31 March 2014.
- ↑ Freezer Baits, nutrabaits.net
- ↑ Kasumyan AO, Døving KB (2003). “Taste preferences in fishes”. 《Fish and Fisheries》 4 (4): 289–347. doi:10.1046/j.1467-2979.2003.00121.x.
- ↑ Japanese Whalers Injured by Acid-Firing Activists, newser.com, 10 February 2010
- ↑ National Abortion Federation, HISTORY OF VIOLENCE Butyric Acid Attacks 보관됨 13 6월 2010 - 웨이백 머신. Prochoice.org. Retrieved on 31 March 2014.
- ↑ Lokensgard, Erik (2015). 《Industrial Plastics: Theory and Applications》 6판. Cengage Learning.
- ↑ Williams, R. Scott. “Care of Plastics: Malignant plastics”. 《WAAC Newsletter》 (Vol. 24, No. 1) (Conservation OnLine). 2017년 5월 29일에 확인함.
- ↑ “Why screwdriver handles smell like vomit and make your tongue numb.”. 《The Garage Journal》. 2017년 5월 29일에 확인함.
- ↑ 가 나 다 Lupton JR (February 2004). “Microbial degradation products influence colon cancer risk: the butyrate controversy”. 《The Journal of Nutrition》 134 (2): 479–82. PMID 14747692.
- ↑ Cummings JH, Macfarlane GT, Englyst HN (February 2001). “Prebiotic digestion and fermentation”. 《The American Journal of Clinical Nutrition》 73 (2 Suppl): 415S–420S. PMID 11157351.
- ↑ Grummer RR (September 1991). “Effect of feed on the composition of milk fat” (PDF). 《Journal of Dairy Science》 74 (9): 3244–57. doi:10.3168/jds.S0022-0302(91)78510-X. PMID 1779073.
- ↑ webmed. “Frequently asked questions in the area of diet and IBS”. 《www.med.monash.edu》. 2016년 3월 24일에 확인함.
- ↑ Gibson, Peter R.; Shepherd, Susan J. (2010년 2월 1일). “Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach”. 《Journal of Gastroenterology and Hepatology》 25 (2): 252–258. doi:10.1111/j.1440-1746.2009.06149.x. ISSN 1440-1746. PMID 20136989.
- ↑ Gibson, Peter R.; Varney, Jane; Malakar, Sreepurna; Muir, Jane G. (2015년 5월 1일). “Food components and irritable bowel syndrome”. 《Gastroenterology》 148 (6): 1158–1174.e4. doi:10.1053/j.gastro.2015.02.005. ISSN 1528-0012. PMID 25680668.
- ↑ Owen, O. E.; Morgan, A. P.; Kemp, H. G.; Sullivan, J. M.; Herrera, M. G.; Cahill, G. F. (1967년 10월 1일). “Brain metabolism during fasting”. 《The Journal of Clinical Investigation》 46 (10): 1589–1595. doi:10.1172/JCI105650. ISSN 0021-9738. PMC 292907. PMID 6061736.
- ↑ Ho, Emily; Clarke, John D.; Dashwood, Roderick H. (2009년 12월 1일). “Dietary Sulforaphane, a Histone Deacetylase Inhibitor for Cancer Prevention”. 《The Journal of Nutrition》 139 (12): 2393–2396. doi:10.3945/jn.109.113332. ISSN 0022-3166. PMC 2777483. PMID 19812222.
- ↑ Pledgie-Tracy, Allison; Sobolewski, Michele D.; Davidson, Nancy E. (2007년 3월 1일). “Sulforaphane induces cell type–specific apoptosis in human breast cancer cell lines”. 《Molecular Cancer Therapeutics》 (영어) 6 (3): 1013–1021. doi:10.1158/1535-7163.MCT-06-0494. ISSN 1535-7163. PMID 17339367.
- ↑ Sasaki, Mari; Shinozaki, Shohei; Shimokado, Kentaro (2016년 3월 25일). “Sulforaphane promotes murine hair growth by accelerating the degradation of dihydrotestosterone”. 《Biochemical and Biophysical Research Communications》 472 (1): 250–254. doi:10.1016/j.bbrc.2016.02.099. ISSN 1090-2104. PMID 26923074.
- ↑ Moon, Joon-Kwan; Kim, Jun-Ran; Ahn, Young-Joon; Shibamoto, Takayuki (2010년 6월 9일). “Analysis and anti-Helicobacter activity of sulforaphane and related compounds present in broccoli ( Brassica oleracea L.) sprouts”. 《Journal of Agricultural and Food Chemistry》 58 (11): 6672–6677. doi:10.1021/jf1003573. ISSN 1520-5118. PMID 20459098.
- ↑ Dash, Pramod K.; Zhao, Jing; Orsi, Sara A.; Zhang, Min; Moore, Anthony N. (2009년 8월 28일). “Sulforaphane improves cognitive function administered following traumatic brain injury”. 《Neuroscience Letters》 460 (2): 103–107. doi:10.1016/j.neulet.2009.04.028. ISSN 1872-7972. PMC 2700200. PMID 19515491.
- ↑ Ghawi, Sameer Khalil; Methven, Lisa; Niranjan, Keshavan. “The potential to intensify sulforaphane formation in cooked broccoli (Brassica oleracea var. italica) using mustard seeds (Sinapis alba)”. 《Food Chemistry》 138 (2–3): 1734–1741. doi:10.1016/j.foodchem.2012.10.119.
- ↑ Yang, C. S.; Chhabra, S. K.; Hong, J. Y.; Smith, T. J. (2001년 3월 1일). “Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic”. 《The Journal of Nutrition》 131 (3s): 1041S–5S. ISSN 0022-3166. PMID 11238812.
- ↑ Sundaram, Sujatha G.; Milner, John A. (1996년 4월 1일). “Diallyl disulfide induces apoptosis of human colon tumor cells”. 《Carcinogenesis》 (영어) 17 (4): 669–673. doi:10.1093/carcin/17.4.669. ISSN 0143-3334. PMID 8625476.
- ↑ 가 나 다 “Butyric acid”. 《IUPHAR》. IUPHAR/BPS Guide to PHARMACOLOGY. 2015년 5월 23일에 확인함.
- ↑ 가 나 “butanoic acid, 4 and Sodium; butyrate”. 《BindingDB》. The Binding Database. 2015년 5월 23일에 확인함.
- ↑ 가 나 다 라 마 바 사 아 자 차 Bourassa MW, Alim I, Bultman SJ, Ratan RR (June 2016). “Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health?”. 《Neurosci. Lett.》 625: 56–63. doi:10.1016/j.neulet.2016.02.009. PMID 26868600.
Butyrate is an attractive therapeutic molecule because of its wide array of biological functions, such as its ability to serve as a histone deacetylase (HDAC) inhibitor, an energy metabolite to produce ATP and a G protein-coupled receptor (GPCR) activator. ... Histone acetylation is a post-translational modification by an epigenetic protein, which are proteins that bind to chromatin and influence chromatin structure to change the propensity that a gene is transcribed or repressed. Acetylated histones cause the chromatin structure to loosen by weakening electrostatic attraction between the histone proteins and the DNA backbone. This process enables transcription factors and the basal transcriptional machinery to bind and increases transcription. ... However, many studies have shown that at least some of these beneficial effects can be attributed NaB’s ability to increase acetylation around the promoters of neurotrophic factors, such as BDNF, GDNF and NGF and thus increasing their transcription [41], [42], [43], [44], [45], [46], [47] and [48]. ... Butyrate also signals through GPR109a ... Much of the butyrate produced in the colon is used as an energy source by the colonocytes, but some butyrate can also exit the colon through the portal vein, where the liver absorbs another large portion [74] and [75]. However, the distal colon is not connected to the portal vein, allowing for some systemic butyrate to be circulated. Indeed, there are many reports of high fiber diets increasing blood levels of circulating butyrate [75], [76] and [77]. These later reports raise the possibility that increases in circulating butyrate could affect CNS function directly.
- ↑ 가 나 다 라 Kasubuchi M, Hasegawa S, Hiramatsu T, Ichimura A, Kimura I (2015). “Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation”. 《Nutrients》 7 (4): 2839–49. doi:10.3390/nu7042839. PMC 4425176. PMID 25875123.
Short-chain fatty acids (SCFAs) such as acetate, butyrate, and propionate, which are produced by gut microbial fermentation of dietary fiber, are recognized as essential host energy sources and act as signal transduction molecules via G-protein coupled receptors (FFAR2, FFAR3, OLFR78, GPR109A) and as epigenetic regulators of gene expression by the inhibition of histone deacetylase (HDAC). Recent evidence suggests that dietary fiber and the gut microbial-derived SCFAs exert multiple beneficial effects on the host energy metabolism not only by improving the intestinal environment, but also by directly affecting various host peripheral tissues.
- ↑ 가 나 다 라 마 바 사 Hoeppli RE, Wu D, Cook L, Levings MK (February 2015). “The environment of regulatory T cell biology: cytokines, metabolites, and the microbiome”. 《Front Immunol》 6: 61. doi:10.3389/fimmu.2015.00061. PMC 4332351. PMID 25741338.
Specific species that have been recognized by their high levels of butyrate production include Faecalibacterium prausnitzii and the cluster IV and XIVa of genus Clostridium ... Administration of acetate, propionate, and butyrate in drinking water mimics the effect of Clostridium colonization in germ-free mice, resulting in an elevated Treg frequency in the colonic lamina propria and increased IL-10 production by these Tregs (180, 182). Of the three main SCFAs, butyrate has been found to be the most potent inducer of colonic Tregs. Mice fed a diet enriched in butyrylated starches have more colonic Tregs than those fed a diet containing propinylated or acetylated starches (181). Arpaia et al. tested an array of SCFAs purified from commensal bacteria and confirmed butyrate was the strongest SCFA-inducer of Tregs in vitro (180). Mechanistically, it has been proposed that butyrate, and possibly propionate, promote Tregs through inhibiting histone deacetylase (HDAC), causing increased acetylation of histone H3 in the Foxp3 CNS1 region, and thereby enhancing FOXP3 expression (180, 181). Short-chain fatty acids partially mediate their effects through G-protein coupled receptors (GPR), including GPR41, GPR43, and GPR109A. GPR41 and GPR43 are stimulated by all three major SCFAs (191), whereas GPR109A only interacts with butyrate (192).
Figure 1: Microbial-derived molecules promote colonic Treg differentiation. - ↑ Tsuji A (2005). “Small molecular drug transfer across the blood-brain barrier via carrier-mediated transport systems”. 《NeuroRx》 2 (1): 54–62. doi:10.1602/neurorx.2.1.54. PMC 539320. PMID 15717057.
Other in vivo studies in our laboratories indicated that several compounds including acetate, propionate, butyrate, benzoic acid, salicylic acid, nicotinic acid, and some β-lactam antibiotics may be transported by the MCT at the BBB.21 ... Uptake of valproic acid was reduced in the presence of medium-chain fatty acids such as hexanoate, octanoate, and decanoate, but not propionate or butyrate, indicating that valproic acid is taken up into the brain via a transport system for medium-chain fatty acids, not short-chain fatty acids.
- ↑ 가 나 Vijay N, Morris ME (2014). “Role of monocarboxylate transporters in drug delivery to the brain”. 《Curr. Pharm. Des.》 20 (10): 1487–98. doi:10.2174/13816128113199990462. PMC 4084603. PMID 23789956.
Monocarboxylate transporters (MCTs) are known to mediate the transport of short chain monocarboxylates such as lactate, pyruvate and butyrate. ... MCT1 and MCT4 have also been associated with the transport of short chain fatty acids such as acetate and formate which are then metabolized in the astrocytes [78]. ... SLC5A8 is expressed in normal colon tissue, and it functions as a tumor suppressor in human colon with silencing of this gene occurring in colon carcinoma. This transporter is involved in the concentrative uptake of butyrate and pyruvate produced as a product of fermentation by colonic bacteria.
- ↑ “triacylglycerol lipase – Homo sapiens”. 《BRENDA》. Technische Universität Braunschweig. 2015년 5월 25일에 확인함.
- ↑ 가 나 다 라 Tilg H, Moschen AR (September 2014). “Microbiota and diabetes: an evolving relationship”. 《Gut》 63 (9): 1513–1521. doi:10.1136/gutjnl-2014-306928. PMID 24833634.
Recent studies have suggested that gut bacteria play a fundamental role in diseases such as obesity, diabetes and cardiovascular disease. Data are accumulating in animal models and humans suggesting that obesity and type 2 diabetes (T2D) are associated with a profound dysbiosis. First human metagenome-wide association studies demonstrated highly significant correlations of specific intestinal bacteria, certain bacterial genes and respective metabolic pathways with T2D. Importantly, especially butyrate-producing bacteria such as Roseburia intestinalis and Faecalibacterium prausnitzii concentrations were lower in T2D subjects. This supports the increasing evidence, that butyrate and other short-chain fatty acids are able to exert profound immunometabolic effects.
- ↑ 가 나 다 라 Wang G (2014). “Human antimicrobial peptides and proteins”. 《Pharmaceuticals (Basel)》 7 (5): 545–94. doi:10.3390/ph7050545. PMC 4035769. PMID 24828484.
The establishment of a link between light therapy, vitamin D and human cathelicidin LL-37 expression provides a completely different way for infection treatment. Instead of treating patients with traditional antibiotics, doctors may be able to use light or vitamin D [291,292]. Indeed using narrow-band UV B light, the level of vitamin D was increased in psoriasis patients (psoriasis is a common autoimmune disease on skin) [293]. In addition, other small molecules such as butyrate can induce LL-37 expression [294]. Components from Traditional Chinese Medicine may regulate the AMP expression as well [295]. These factors may induce the expression of a single peptide or multiple AMPs [296]. It is also possible that certain factors can work together to induce AMP expression. While cyclic AMP and butyrate synergistically stimulate the expression of chicken β-defensin 9 [297], 4-phenylbutyrate (PBA) and 1,25-dihydroxyvitamin D3 (or lactose) can induce AMP gene expression synergistically [294,298]. It appears that stimulation of LL-37 expression by histone deacetylase (HDAC) inhibitors is cell dependent. Trichostatin and sodium butyrate increased the peptide expression in human NCI-H292 airway epithelial cells but not in the primary cultures of normal nasal epithelial cells [299]. However, the induction of the human LL-37 expression may not be a general approach for bacterial clearance. During Salmonella enterica infection of human monocyte-derived macrophages, LL-37 is neither induced nor required for bacterial clearance [300].
Table 3: Select human antimicrobial peptides and their proposed targets
Table 4: Some known factors that induce antimicrobial peptide expression - ↑ Yonezawa H, Osaki T, Hanawa T, Kurata S, Zaman C, Woo TD, Takahashi M, Matsubara S, Kawakami H, Ochiai K, Kamiya S (2012). “Destructive effects of butyrate on the cell envelope of Helicobacter pylori”. 《J. Med. Microbiol.》 61 (Pt 4): 582–9. doi:10.1099/jmm.0.039040-0. PMID 22194341.
- ↑ McGee DJ, George AE, Trainor EA, Horton KE, Hildebrandt E, Testerman TL (2011). “Cholesterol enhances Helicobacter pylori resistance to antibiotics and LL-37”. 《Antimicrob. Agents Chemother.》 55 (6): 2897–904. doi:10.1128/AAC.00016-11. PMC 3101455. PMID 21464244.
- ↑ 가 나 Zimmerman MA, Singh N, Martin PM, Thangaraju M, Ganapathy V, Waller JL, Shi H, Robertson KD, Munn DH, Liu K (2012). “Butyrate suppresses colonic inflammation through HDAC1-dependent Fas upregulation and Fas-mediated apoptosis of T cells”. 《Am. J. Physiol. Gastrointest. Liver Physiol.》 302 (12): G1405–15. doi:10.1152/ajpgi.00543.2011. PMC 3378095. PMID 22517765.
- ↑ Offermanns S, Schwaninger M (2015). “Nutritional or pharmacological activation of HCA(2) ameliorates neuroinflammation”. 《Trends Mol Med》 21 (4): 245–255. doi:10.1016/j.molmed.2015.02.002. PMID 25766751.
Neuroinflammatory cells express HCA2, a receptor for the endogenous neuroprotective ketone body β-hydroxybutyrate (BHB) as well as for the drugs dimethyl fumarate (DMF) and nicotinic acid, which have established efficacy in the treatment of MS and experimental stroke, respectively. This review summarizes the evidence that HCA2 is involved in the therapeutic effects of DMF, nicotinic acid, and ketone bodies in reducing neuroinflammation.
- ↑ Chai JT, Digby JE, Choudhury RP (May 2013). “GPR109A and vascular inflammation”. 《Curr Atheroscler Rep》 15 (5): 325. doi:10.1007/s11883-013-0325-9. PMC 3631117. PMID 23526298.
As GPR109A's primary pharmacological ligand in clinical use, niacin has been used for over 50 years in the treatment of cardiovascular disease, mainly due to its favourable effects on plasma lipoproteins. However, it has become apparent that niacin also possesses lipoprotein-independent effects that influence inflammatory pathways mediated through GPR109A.
- ↑ Graff EC, Fang H, Wanders D, Judd RL (February 2016). “Anti-inflammatory effects of the hydroxycarboxylic acid receptor 2”. 《Metab. Clin. Exp.》 65 (2): 102–113. doi:10.1016/j.metabol.2015.10.001. PMID 26773933.
HCA2 is highly expressed on immune cells, including macrophages, monocytes, neutrophils and dermal dendritic cells, among other cell types. ... Recent studies demonstrate that HCA2 mediates profound anti-inflammatory effects in a variety of tissues, indicating that HCA2 may be an important therapeutic target for treating inflammatory disease processes.
- ↑ Wakade C, Chong R (December 2014). “A novel treatment target for Parkinson's disease”. 《J. Neurol. Sci.》 347 (1-2): 34–38. doi:10.1016/j.jns.2014.10.024. PMID 25455298.
GPR109A and its agonists are known to exert anti-inflammatory actions in the skin, gut and retina.
- ↑ Farzi A, Reichmann F, Holzer P (2015). “The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour”. 《Acta Physiol (Oxf)》 213 (3): 603–27. doi:10.1111/apha.12445. PMC 4353849. PMID 25545642.
In the context of this review it is particularly worth noting that short chain fatty acids such as butyrate, which the colonic microbiota generates by fermentation of otherwise indigestible dietary fibre (Cherbut et al. 1998), stimulate L cells to release PYY via the G-protein coupled receptor Gpr41 (Samuel et al. 2008). In this way, short chain fatty acids can indirectly attenuate gastrointestinal motility as well as electrolyte and water secretion (Cox 2007b). More importantly, short chain fatty acids exert homeostatic actions on the function of the colonic mucosa and immune system (Hamer et al. 2008, Tazoe et al. 2008, Guilloteau et al. 2010, Macia et al. 2012a, Smith et al. 2013). Whether PYY plays a role in these effects of short chain fatty acids awaits to be investigated, but may be envisaged from the finding that PYY promotes mucosal cell differentiation (Hallden & Aponte 1997).
- ↑ Donohoe, Dallas R.; Garge, Nikhil; Zhang, Xinxin; Sun, Wei; O’Connell, Thomas M.; Bunger, Maureen K.; Bultman, Scott J. (2011년 5월 4일). “The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon”. 《Cell Metabolism》 13 (5): 517–526. doi:10.1016/j.cmet.2011.02.018. ISSN 1550-4131. PMC 3099420. PMID 21531334.
- ↑ Vanhoutvin SA, Troost FJ, Hamer HM, Lindsey PJ, Koek GH, Jonkers DM, Kodde A, Venema K, Brummer RJ (2009). Bereswill S, 편집. “Butyrate-induced transcriptional changes in human colonic mucosa”. 《PLOS ONE》 4 (8): e6759. doi:10.1371/journal.pone.0006759. PMC 2727000. PMID 19707587.[깨진 링크]
- ↑ Klampfer L, Huang J, Sasazuki T, Shirasawa S, Augenlicht L (August 2004). “Oncogenic Ras promotes butyrate-induced apoptosis through inhibition of gelsolin expression” (PDF). 《The Journal of Biological Chemistry》 279 (35): 36680–8. doi:10.1074/jbc.M405197200. PMID 15213223.
- ↑ Lupton, Joanne R. (2004). 《Microbial Degradation Products Influence Colon Cancer Risk: the Butyrate Controversy》. vol. 134 no. 2: J. Nutr. 479–482쪽.
- ↑ “Low-carb diet cuts risk of colon cancer, study finds | University of Toronto Media Room”. 《media.utoronto.ca》. 2016년 5월 4일에 확인함.
- ↑ Belcheva, Antoaneta; Irrazabal, Thergiory; Robertson, Susan J.; Streutker, Catherine; Maughan, Heather; Rubino, Stephen; Moriyama, Eduardo H.; Copeland, Julia K.; Kumar, Sachin (2014년 7월 17일). “Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells”. 《Cell》 158 (2): 288–299. doi:10.1016/j.cell.2014.04.051. ISSN 1097-4172. PMID 25036629.
- ↑ “Colon Cancer Shuts Down Receptor That Could Shut It Down”. 《ScienceDaily》. 2016년 5월 4일에 확인함.
- ↑ Davie, James R. (2003년 7월 1일). “Inhibition of Histone Deacetylase Activity by Butyrate”. 《The Journal of Nutrition》 (영어) 133 (7): 2485S–2493S. ISSN 0022-3166. PMID 12840228.
- ↑ Cadet, Jean Lud; Brannock, Christie; Jayanthi, Subramaniam; Krasnova, Irina N. (2015년 1월 1일). “Transcriptional and Epigenetic Substrates of Methamphetamine Addiction and Withdrawal: Evidence from a Long-Access Self-Administration Model in the Rat”. 《Molecular Neurobiology》 51 (2): 696–717. doi:10.1007/s12035-014-8776-8. ISSN 0893-7648. PMC 4359351. PMID 24939695.
- ↑ Kennedy, Pamela J.; Feng, Jian; Robison, A.J.; Maze, Ian; Badimon, Ana; Mouzon, Ezekiell; Chaudhury, Dipesh; Damez-Werno, Diane M.; Haggarty, Stephen J. (2013년 4월 1일). “Class I HDAC Inhibition Blocks Cocaine-Induced Plasticity Through Targeted Changes in Histone Methylation”. 《Nature Neuroscience》 16 (4): 434–440. doi:10.1038/nn.3354. ISSN 1097-6256. PMC 3609040. PMID 23475113.
- ↑ Malvaez, Melissa; McQuown, Susan C.; Rogge, George A.; Astarabadi, Mariam; Jacques, Vincent; Carreiro, Samantha; Rusche, James R.; Wood, Marcelo A. (2013년 2월 12일). “HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner”. 《Proceedings of the National Academy of Sciences of the United States of America》 110 (7): 2647–2652. doi:10.1073/pnas.1213364110. ISSN 0027-8424. PMC 3574934. PMID 23297220.
- ↑ Romieu, Pascal; Host, Lionel; Gobaille, Serge; Sandner, Guy; Aunis, Dominique; Zwiller, Jean (2008년 9월 17일). “Histone deacetylase inhibitors decrease cocaine but not sucrose self-administration in rats”. 《The Journal of Neuroscience》 28 (38): 9342–9348. doi:10.1523/JNEUROSCI.0379-08.2008. ISSN 1529-2401. PMID 18799668.
- ↑ Bravo, Javier A.; Forsythe, Paul; Chew, Marianne V.; Escaravage, Emily; Savignac, Hélène M.; Dinan, Timothy G.; Bienenstock, John; Cryan, John F. (2011년 9월 20일). “Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve”. 《Proceedings of the National Academy of Sciences》 (영어) 108 (38): 16050–16055. doi:10.1073/pnas.1102999108. ISSN 0027-8424. PMC 3179073. PMID 21876150.
- ↑ 《The Developing Genome: An Introduction to Behavioral Epigenetics》 (영어) 1판. Oxford University Press. 2015년 3월 2일. ISBN 9780199922345.
- ↑ Fischer, Andre; Sananbenesi, Farahnaz; Wang, Xinyu; Dobbin, Matthew; Tsai, Li-Huei (2007년 5월 10일). “Recovery of learning and memory is associated with chromatin remodelling”. 《Nature》 447 (7141): 178–182. doi:10.1038/nature05772. ISSN 1476-4687. PMID 17468743.
- ↑ 가 나 Miller, Greg (2010년 7월 2일). “Epigenetics. The seductive allure of behavioral epigenetics”. 《Science》 329 (5987): 24–27. doi:10.1126/science.329.5987.24. ISSN 1095-9203. PMID 20595592.
- ↑ Gupta, Swati; Kim, Se Y.; Artis, Sonja; Molfese, David L.; Schumacher, Armin; Sweatt, J. David; Paylor, Richard E.; Lubin, Farah D. (2010년 3월 10일). “Histone methylation regulates memory formation”. 《The Journal of Neuroscience》 30 (10): 3589–3599. doi:10.1523/JNEUROSCI.3732-09.2010. ISSN 1529-2401. PMC 2859898. PMID 20219993.
- ↑ Peleg, Shahaf; Sananbenesi, Farahnaz; Zovoilis, Athanasios; Burkhardt, Susanne; Bahari-Javan, Sanaz; Agis-Balboa, Roberto Carlos; Cota, Perla; Wittnam, Jessica Lee; Gogol-Doering, Andreas (2010년 5월 7일). “Altered histone acetylation is associated with age-dependent memory impairment in mice”. 《Science》 328 (5979): 753–756. doi:10.1126/science.1186088. ISSN 1095-9203. PMID 20448184.
- ↑ Gräff, Johannes; Rei, Damien; Guan, Ji-Song; Wang, Wen-Yuan; Seo, Jinsoo; Hennig, Krista M.; Nieland, Thomas J. F.; Fass, Daniel M.; Kao, Patricia F. (2012년 3월 8일). “An epigenetic blockade of cognitive functions in the neurodegenerating brain”. 《Nature》 483 (7388): 222–226. doi:10.1038/nature10849. ISSN 1476-4687. PMC 3498952. PMID 22388814.
External links
- International Chemical Safety Card 1334
- 2004 review of the scientific evidence on butanoate/butyrate vs. colon cancer
틀:Fatty acids 틀:HDAC inhibitors 틀:GABA metabolism and transport modulators
