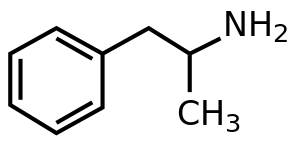
암페타민
| |

| |
| 체계적 명칭 (IUPAC 명명법) | |
|---|---|
| 식별 정보 | |
| CAS 등록번호 | 300-62-9 |
| ATC 코드 | N06BA01 |
| PubChem | 3007 |
| 드러그뱅크 | DB00182 |
| ChemSpider | 13852819 |
| 화학적 성질 | |
| 화학식 | C9H13N |
| 분자량 | 135.20622 g/mol[1] |
| SMILES | eMolecules & PubChem |
| 유의어 | α-methylphenethylamine |
| 물리적 성질 | |
| 밀도 | .936 g/cm³ |
| 녹는점 | 11.3 °C (52 °F) (predicted)[2] |
| 끓는점 | 203 °C (397 °F) at 760 mmHg[3] |
| 약동학 정보 | |
| 생체적합성 | oral: 75–100%[4] |
| 단백질 결합 | 15–40%[5] |
| 동등생물의약품 | ? |
| 약물 대사 | CYP2D6,[6] DBH,[7][8] FMO3[7][9][10] |
| 생물학적 반감기 | D-amph: 9–11 hours[6][11] L-amph: 11–14 hours[6][11] pH-dependent: 7–34 hours[12] |
| 배출 | Primarily renal; pH-dependent range: 1–75%[6] |
| 처방 주의사항 | |
| 임부투여안전성 | C(미국) |
| 법적 상태 |
|
| 중독 경향 | 신체적 의존성: 없음[13] 정신적 의존성: moderate[14] |
| 투여 방법 | 경구 |
암페타민(Amphetamine, alpha-methylphenethylamine의 준말)은 피로와 식욕을 낮추고 기민성을 증가시키는 펜에틸아민 계열의 중추신경계 각성제의 일종으로, 주로 주의력결핍 과다행동장애(ADHD), 기면증, 비만증 등의 치료제로 쓰인다.[15]
노르에피네프린 및 도파민의 재흡수 억제제로 작용하여 (NDRI) 각성 효과를 일으키며, 특히 도파민의 분비를 촉진하고 관련 신경전달계를 개선하여 ADHD의 증상 완화 및 치료에 탁월한 효과를 보인다. 또한 일시적으로 반응속도, 근력의 향상을 보여 경기력 향상 약물로 남용되기도 했다.
역사적으로는 1887년 발견된 이후 비충혈 제거제로서 의학적으로 쓰여왔으며 우울증 치료제로도 사용되어 왔다.[15][16][17] 정신적 의존이 나타날 수 있어 한국, 일본 등 일부 국가에서는 사용이 금지되어 있으나 이들을 제외한 전 세계 183개국에서 의료적 처방이 승인되어 있다.[18] 약의 상표명으로 에베케오(Evekeo), 애더럴(Adderall) 등이 있다.
합성[편집]
약품명[편집]
- - 벤제드린(Benzedrine)
- - 이브케오(evekeo)
- -바아반스(vyvanse)
- -젠데디 (zendedi)
- -덱시드린(Dexedrine)
같이 보기[편집]
참조[편집]
- ↑ 〈Compound Summary〉. 《Amphetamine》. 《PubChem Compound Database》. United States National Library of Medicine – National Center for Biotechnology Information. 2015년 4월 11일. 2015년 4월 17일에 확인함.
- ↑ 〈Properties: Predicted – EPISuite〉. 《Amphetamine》. 《ChemSpider》. Royal Society of Chemistry. 2013년 11월 6일에 확인함.
- ↑ 〈Chemical and Physical Properties〉. 《Amphetamine》. 《PubChem Compound Database》. United States National Library of Medicine – National Center for Biotechnology Information. 2013년 10월 13일에 확인함.
- ↑ 〈Pharmacology〉. 《Dextroamphetamine》. 《DrugBank》. University of Alberta. 2013년 2월 8일. 2013년 11월 5일에 확인함.
- ↑ 〈Pharmacology〉. 《Amphetamine》. 《DrugBank》. University of Alberta. 2013년 2월 8일. 2013년 11월 5일에 확인함.
- ↑ 가 나 다 라 “Adderall XR Prescribing Information” (PDF). 《United States Food and Drug Administration》. Shire US Inc. December 2013. 12–13쪽. 2013년 12월 30일에 확인함.
- ↑ 가 나 Glennon RA (2013). 〈Phenylisopropylamine stimulants: amphetamine-related agents〉. Lemke TL, Williams DA, Roche VF, Zito W. 《Foye's principles of medicinal chemistry》 7판. Philadelphia, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins. 646–648쪽. ISBN 9781609133450.
- ↑ Taylor KB (January 1974). “Dopamine-beta-hydroxylase. Stereochemical course of the reaction” (PDF). 《J. Biol. Chem.》 249 (2): 454–458. PMID 4809526. 2014년 11월 6일에 확인함.
- ↑ Krueger SK, Williams DE (June 2005). “Mammalian flavin-containing monooxygenases: structure/function, genetic polymorphisms and role in drug metabolism”. 《Pharmacol. Ther.》 106 (3): 357–387. doi:10.1016/j.pharmthera.2005.01.001. PMC 1828602. PMID 15922018.
Table 5: N-containing drugs and xenobiotics oxygenated by FMO - ↑ Cashman JR, Xiong YN, Xu L, Janowsky A (March 1999). “N-oxygenation of amphetamine and methamphetamine by the human flavin-containing monooxygenase (form 3): role in bioactivation and detoxication”. 《J. Pharmacol. Exp. Ther.》 288 (3): 1251–1260. PMID 10027866.
- ↑ 가 나 “Adderall IR Prescribing Information” (PDF). 《United States Food and Drug Administration》. Teva Pharmaceuticals USA, Inc. October 2015. 1–6쪽. 2016년 5월 18일에 확인함.
- ↑ 〈Metabolism/Pharmacokinetics〉. 《Amphetamine》. 《United States National Library of Medicine – Toxicology Data Network》. Hazardous Substances Data Bank. 2017년 10월 2일에 원본 문서에서 보존된 문서. 2017년 10월 2일에 확인함.
- ↑ Malenka RC, Nestler EJ, Hyman SE, Holtzman DM (2015). 〈Chapter 16: Reinforcement and Addictive Disorders〉. 《Molecular Neuropharmacology: A Foundation for Clinical Neuroscience》 3판. New York: McGraw-Hill Medical. ISBN 9780071827706.
- ↑ Stahl SM (March 2017). 〈Amphetamine (D,L)〉. 《Prescriber's Guide: Stahl's Essential Psychopharmacology》 6판. Cambridge, United Kingdom: Cambridge University Press. 45–51쪽. ISBN 9781108228749. 2017년 8월 5일에 확인함.
- ↑ 가 나 Heal DJ, Smith SL, Gosden J, Nutt DJ (2013년 6월). “Amphetamine, past and present – a pharmacological and clinical perspective”. 《J. Psychopharmacol.》 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
- ↑ Rasmussen N (2006년 7월). “Making the first anti-depressant: amphetamine in American medicine, 1929–1950”. 《J . Hist. Med. Allied Sci.》 61 (3): 288–323. doi:10.1093/jhmas/jrj039. PMID 16492800.
- ↑ “Methamphetamine facts”. 《DrugPolicy.org》. 2013년 10월 19일에 확인함.
- ↑ “Convention on psychotropic substances”. 《United Nations Treaty Collection》. United Nations. 2016년 3월 31일에 원본 문서에서 보존된 문서. 2013년 11월 11일에 확인함.



