프로게스테론: 두 판 사이의 차이
내용 삭제됨 내용 추가됨
잔글 +분류:프레그난 |
편집 요약 없음 |
||
| 1번째 줄: | 1번째 줄: | ||
{{Chembox |
|||
| ⚫ | |||
<!-- Images -->| Name = |
|||
| ImageFile = |
|||
| ⚫ | |||
| ImageSize1 = 225px |
|||
| ImageAlt1 = The chemical structure of progesterone. |
|||
| ⚫ | |||
| ImageSize2 = 225px |
|||
| ImageAlt2 = A ball-and-stick model of progesterone. |
|||
<!-- Names -->| IUPACName = (8''S'',9''S'',10''R'',13''S'',14''S'',17''S'')-17-acetyl-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one |
|||
| OtherNames = P4;<ref name=James2015/> Pregnenedione; Pregn-4-ene-3,20-dione<ref>{{cite book | first1 = Norman | last1 = Adler | first2 = Donald | last2 = Pfaff | first3 = Robert W. | last3 = Goy | name-list-style = vanc | title = Handbook of Behavioral Neurobiology Volume 7 Reproduction | date = 6 Dec 2012 | publisher=Plenum Press | location = New York | isbn = 978-1-4684-4834-4 | page = 189 | edition = 1st | url = https://books.google.com/books?id=MoDrBwAAQBAJ&q=pregn-4-ene-3,20-dione;+abbreviated+as+P4&pg=PA189 | accessdate = 4 July 2015 }}</ref><ref>{{cite web|title=progesterone (CHEBI:17026)|url=http://www.ebi.ac.uk/chebi/searchId.do;jsessionid=309FCC7D184C0AD58410071F3F163155?chebiId=17026&structureView=applet&viewTermLineage=|website=ChEBI|publisher=European Molecular Biology Laboratory-EBI|accessdate=4 July 2015}}</ref> |
|||
| Verifiedfields = changed |
|||
| Watchedfields = changed |
|||
| verifiedrevid = 444066687 |
|||
<!-- Sections -->| SystematicName = |
|||
| Section1 = {{Chembox Identifiers |
|||
| CASNo_Ref = {{cascite|correct|??}} |
|||
| CASNo = 57-83-0 |
|||
| ChEBI_Ref = {{ebicite|correct|EBI}} |
|||
| ChEBI = 17026 |
|||
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|||
| ChEMBL = 103 |
|||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|||
| ChemSpiderID = 5773 |
|||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
|||
| DrugBank = DB00396 |
|||
| KEGG_Ref = {{keggcite|changed|kegg}} |
|||
| KEGG = C00410 |
|||
| PubChem = 5994 |
|||
| SMILES = CC(=O)[C@H]1CC[C@@H]2[C@@]1(CC[C@H]3[C@H]2CCC4=CC(=O)CC[C@]34C)C |
|||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|||
| StdInChI = InChI=1S/C21H30O2/c1-13(22)17-6-7-18-16-5-4-14-12-15(23)8-10-20(14,2)19(16)9-11-21(17,18)3/h12,16-19H,4-11H2,1-3H3/t16-,17+,18-,19-,20-,21+/m0/s1 |
|||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|||
| StdInChIKey = RJKFOVLPORLFTN-LEKSSAKUSA-N |
|||
| UNII_Ref = {{fdacite|correct|FDA}} |
|||
| UNII = 4G7DS2Q64Y |
|||
}} |
|||
| Section2 = {{Chembox Properties |
|||
| C=21 | H=30 | O=2 |
|||
| MolarMass = 314.469 g/mol |
|||
| Appearance = |
|||
| Density = |
|||
| MeltingPt = 126 |
|||
| BoilingPt = |
|||
| Solubility = |
|||
| LogP = 4.04<ref name="chemsrc">{{Cite web|url=https://www.chemsrc.com/en/cas/57-83-0_1068061.html|title=Progesterone_msds}}</ref> |
|||
}} |
|||
| Section3 = {{Chembox Hazards |
|||
| MainHazards = |
|||
| FlashPt = |
|||
| AutoignitionPt = |
|||
}} |
|||
| Section4 = |
|||
| Section5 = |
|||
| Section6 = {{Chembox Pharmacology |
|||
| ATCvet = |
|||
| ATCCode_prefix = G03 |
|||
| ATCCode_suffix = DA04 |
|||
| ATC_Supplemental = |
|||
| AdminRoutes = [[Oral administration|By mouth]], [[topical medication|topical]]/[[transdermal]], [[intravaginal administration|vaginal]], [[intramuscular injection]], [[subcutaneous injection]], [[implant (medicine)|subcutaneous implant]] |
|||
| Bioavail = {{abbr|OMP|oral micronized progesterone}}: <10%<ref name="Stanczyk2002">{{cite journal | vauthors = Stanczyk FZ | title = Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception | journal = Reviews in Endocrine & Metabolic Disorders | volume = 3 | issue = 3 | pages = 211–24 | date = September 2002 | pmid = 12215716 | doi = 10.1023/A:1020072325818 | s2cid = 27018468 }}</ref><ref name="SimonRobinson1993">{{cite journal | vauthors = Simon JA, Robinson DE, Andrews MC, Hildebrand JR, Rocci ML, Blake RE, Hodgen GD | title = The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone | journal = Fertility and Sterility | volume = 60 | issue = 1 | pages = 26–33 | date = July 1993 | pmid = 8513955 | doi = 10.1016/S0015-0282(16)56031-2 }}</ref> |
|||
| Excretion = [[Renal]] |
|||
| HalfLife = {{abbr|OMP|oral micronized progesterone}}: 16–18 hours<ref name="Stanczyk2002" /><ref name="SimonRobinson1993" /><ref name="Zutshi2005">{{cite book | author = Zutshi | title = Hormones in Obstetrics and Gynaecology | url = https://books.google.com/books?id=IBxBbaDjXw0C&pg=PA74 | date = 1 January 2005 | publisher = Jaypee Brothers Publishers | isbn = 978-81-8061-427-9 | page = 74}}</ref><br />{{abbr|IM|Intramuscular}}: 22–26 hours<ref name="SimonRobinson1993" /><ref name="Cometti2015">{{cite journal | vauthors = Cometti B | title = Pharmaceutical and clinical development of a novel progesterone formulation | journal = Acta Obstetricia et Gynecologica Scandinavica | volume = 94 | issue = Suppl 161 | pages = 28–37 | date = November 2015 | pmid = 26342177 | doi = 10.1111/aogs.12765 | s2cid = 31974637 }}</ref><br />{{abbr|SC|Subcutaneous}}: 13–18 hours<ref name="Cometti2015" /> |
|||
| Metabolism = [[Hepatic]] ([[CYP2C19]], [[CYP3A4]], [[CYP2C9]], [[5α-reductase]], {{abbrlink|3α-HSD|3α-hydroxysteroid dehydrogenase}}, [[17α-hydroxylase]], [[21-hydroxylase]], {{abbrlink|20α-HSD|20α-hydroxysteroid dehydrogenase}})<ref name="pmid9328296">{{cite journal | vauthors = Yamazaki H, Shimada T | title = Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes | journal = Archives of Biochemistry and Biophysics | volume = 346 | issue = 1 | pages = 161–9 | date = October 1997 | pmid = 9328296 | doi = 10.1006/abbi.1997.0302 }}</ref><ref name="McKayWalters2013">{{cite book | first1 = Gerard A. | last1 = McKay | first2 = Matthew R. | last2 = Walters | name-list-style = vanc | title = Lecture Notes: Clinical Pharmacology and Therapeutics | url = https://books.google.com/books?id=OGOqcfN_Cc8C&pg=PT33 | date = 6 February 2013 | publisher = John Wiley & Sons | isbn = 978-1-118-34489-7 | page = 33}}</ref> |
|||
| ProteinBound = • [[Human serum albumin|Albumin]]: 80%<br />• [[Corticosteroid-binding globulin|CBG]]: 18%<br />• [[Sex hormone-binding globulin|SHBG]]: <1%<br />• Free: 1–2%<ref name="FritzSperoff2012">{{cite book | first1 = Marc A. | last1 = Fritz | first2 = Leon | last2 = Speroff | name-list-style = vanc | title = Clinical Gynecologic Endocrinology and Infertility | url = https://books.google.com/books?id=KZLubBxJEwEC&pg=PA44 | date = 28 March 2012 | publisher = Lippincott Williams & Wilkins | isbn = 978-1-4511-4847-3 | pages = 44– }}</ref><ref name="MarshallD.2008">{{cite book | first1 = William J. | last1 = Marshall | first2 = William J. | last2 = Marshall | first3 = S. K. | last3 = Bangert | name-list-style = vanc | title = Clinical Chemistry | url = https://books.google.com/books?id=Gjc704GR5YEC&pg=PA192 | year = 2008 | publisher = Elsevier Health Sciences|isbn=978-0-7234-3455-9|pages=192–}}</ref> |
|||
}} |
|||
}} |
|||
| ⚫ | |||
| ⚫ | |||
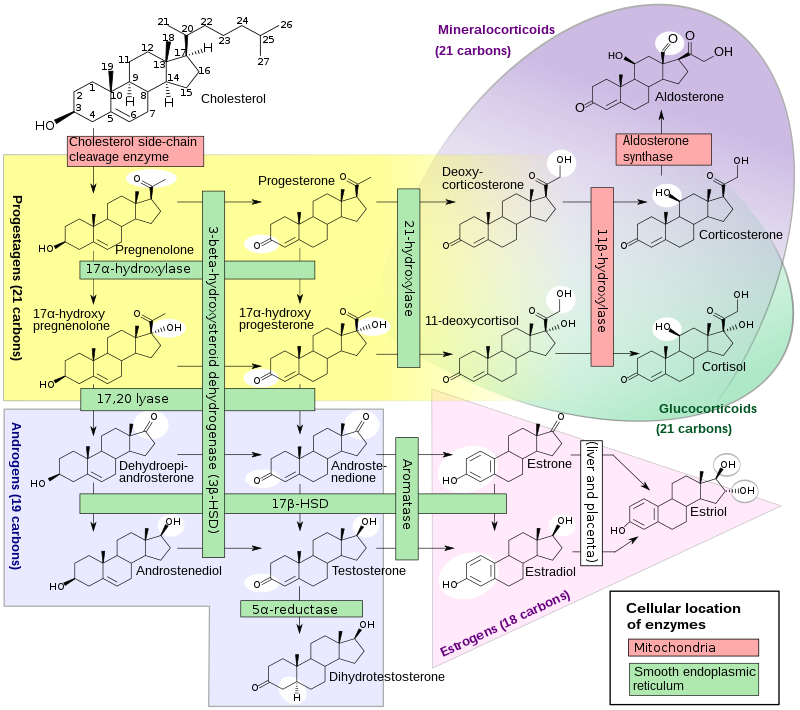
= 화학 구조 = |
|||
| ⚫ | |||
| ⚫ | |||
| ⚫ | |||
[[파일:Steroidogenesis.svg|800px]] |
[[파일:Steroidogenesis.svg|800px]] |
||
= |
== 같이 보기 == |
||
* [[에스트로겐]](estrogen) |
* [[에스트로겐]](estrogen) |
||
== 각주 == |
|||
{{각주}} |
|||
[[분류:성호르몬]] |
[[분류:성호르몬]] |
||
2020년 11월 4일 (수) 04:28 판

| |

| |
| 이름 | |
|---|---|
| IUPAC 이름
(8S,9S,10R,13S,14S,17S)-17-acetyl-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one
| |
| 별칭 | |
| 식별자 | |
3D 모델 (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.318 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| 성질 | |
| C21H30O2 | |
| 몰 질량 | 314.469 g/mol |
| 녹는점 | 126 |
| log P | 4.04[4] |
| 약리학 | |
| G03DA04 (WHO) | |
| By mouth, topical/transdermal, vaginal, intramuscular injection, subcutaneous injection, subcutaneous implant | |
| 약물동태학: | |
| OMP: <10%[5][6] | |
| • Albumin: 80% • CBG: 18% • SHBG: <1% • Free: 1–2%[7][8] | |
| Hepatic (CYP2C19, CYP3A4, CYP2C9, 5α-reductase, 3α-HSD, 17α-hydroxylase, 21-hydroxylase, 20α-HSD)[9][10] | |
| OMP: 16–18 hours[5][6][11] IM: 22–26 hours[6][12] SC: 13–18 hours[12] | |
| Renal | |
달리 명시된 경우를 제외하면, 표준상태(25 °C [77 °F], 100 kPa)에서 물질의 정보가 제공됨.
| |
프로게스테론(progesterone, P4)은 여성의 호르몬 중 하나이다. 여포 자극 호르몬(follicle stimulating hormone, FSH), 황체형성 호르몬(luteinizing hormone, LH), 에스트로겐(estrogen)과 함께 여성의 생식 주기를 조절한다. 월경 시작일 기준으로 1~14일을 여포기(follicular phase), 14~28일을 황체기(luteal phase)라고 한다.
생화학적 합성 경로
같이 보기
- 에스트로겐(estrogen)
각주
- ↑ 인용 오류:
<ref>태그가 잘못되었습니다;James2015라는 이름을 가진 주석에 텍스트가 없습니다 - ↑ Adler, Norman; Pfaff, Donald; Goy, Robert W. (2012년 12월 6일). 《Handbook of Behavioral Neurobiology Volume 7 Reproduction》 1판. New York: Plenum Press. 189쪽. ISBN 978-1-4684-4834-4. 2015년 7월 4일에 확인함.
- ↑ “progesterone (CHEBI:17026)”. 《ChEBI》. European Molecular Biology Laboratory-EBI. 2015년 7월 4일에 확인함.
- ↑ “Progesterone_msds”.
- ↑ 가 나 Stanczyk FZ (September 2002). “Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception”. 《Reviews in Endocrine & Metabolic Disorders》 3 (3): 211–24. doi:10.1023/A:1020072325818. PMID 12215716. S2CID 27018468.
- ↑ 가 나 다 Simon JA, Robinson DE, Andrews MC, Hildebrand JR, Rocci ML, Blake RE, Hodgen GD (July 1993). “The absorption of oral micronized progesterone: the effect of food, dose proportionality, and comparison with intramuscular progesterone”. 《Fertility and Sterility》 60 (1): 26–33. doi:10.1016/S0015-0282(16)56031-2. PMID 8513955.
- ↑ Fritz, Marc A.; Speroff, Leon (2012년 3월 28일). 《Clinical Gynecologic Endocrinology and Infertility》. Lippincott Williams & Wilkins. 44–쪽. ISBN 978-1-4511-4847-3.
- ↑ Marshall, William J.; Marshall, William J.; Bangert, S. K. (2008). 《Clinical Chemistry》. Elsevier Health Sciences. 192–쪽. ISBN 978-0-7234-3455-9.
- ↑ Yamazaki H, Shimada T (October 1997). “Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes”. 《Archives of Biochemistry and Biophysics》 346 (1): 161–9. doi:10.1006/abbi.1997.0302. PMID 9328296.
- ↑ McKay, Gerard A.; Walters, Matthew R. (2013년 2월 6일). 《Lecture Notes: Clinical Pharmacology and Therapeutics》. John Wiley & Sons. 33쪽. ISBN 978-1-118-34489-7.
- ↑ Zutshi (2005년 1월 1일). 《Hormones in Obstetrics and Gynaecology》. Jaypee Brothers Publishers. 74쪽. ISBN 978-81-8061-427-9.
- ↑ 가 나 Cometti B (November 2015). “Pharmaceutical and clinical development of a novel progesterone formulation”. 《Acta Obstetricia et Gynecologica Scandinavica》 94 (Suppl 161): 28–37. doi:10.1111/aogs.12765. PMID 26342177. S2CID 31974637.