사용자:Astronauthang/엔트로피

엔트로피는 에너지 전환, 엔진, 기계와 같은 것에서 일어나는 열역학적 과정에서 일로써 사용불가능한 에너지를 결정하는데 쓰이는 열역학적 특성이다. 이러한 장치들은 사용가능한 에너지에 의해서만 작동이 될 수 있으며 또한 에너지가 일로 전환될 때 이론적인 최대 효율을 지닌다. 이러한 일이 진행될 때, 계의 엔트로피는 증가한다.
엔트로피의 개념은 고립계의 엔트로피는 항상 증가하거나 일정하게 유지된다는 법칙인 열역학 제2법칙에 의해 정의된다. 따라서 엔트로피는 과정이 진행되는 방향과 같은 성질을 나타내는 척도이다. 이것은 열에너지는 항상 열의 형태로 높은 온도인 곳에서 낮은 온도인 곳으로 자발적으로 흘러가는 것을 설명한다. 이러한 과정은 초기 계의 배열의 상태를 줄이게 된다. 따라서 엔트로피는 무질서의 정도를 나타낸다. 이 모델은 가능한 양자상태에 있는 열역학 계의 구성 요소들의 확률을 기술하는 통계역학적 엔트로피의 미시적 관점의 해석에 기초하고 있다. 이는 정보 엔트로피의 개념과 직접적으로 연관된다.
열역학적 엔트로피의 차원은 에너지 나누기 온도이며 단위는 국제단위계로 줄 나누기 켈빈 (J/K) 이다.
‘엔트로피’라는 단어는 1865년 루돌프 클라우지우스가 그리스어로 ‘회전’이란 의미의 εντροπία [entropía]에 기초하여 만들었다.
열역학적 표현과 통계학적 표현[편집]
엔트로피를 정의하는 방법에는 열역학적 정의와 통계역학적 정의가 있다. 열역학적 정의는 1850년대 초 루돌프 클라우지우스에 의해 발전되었고 열적 평형상태에서 고립계의 엔트로피를 측정하는 방법을 기술한다. 특히 이것은 물질의 미시적 관점에 대한 언급이 없다. 통계역학적 정의는 1870년대에 루트비히 볼츠만에 의해 계의 미시 성분들의 통계적 특성의 분석을 통해 발전되었다. 볼츠만은 통계역학적으로 정의된 엔트로피와 열역학적으로 정의된 엔트로피가 볼츠만 상수로 알려진 상수를 를 통해 동일하다는 사실을 보였다.
열역학적 엔트로피는 비보존 상태함수이다. 역사적으로 엔트로피의 개념은 몇몇 자발적으로 일어나는 과정의 역과정이 비자발적인 이유에 대해 설명하고자 발전하였다 (과정과 역과정 모두 보존법칙들이 성립). 고립계에서 엔트로피는 절대 감소하지 않는다. 이 사실은 과학에서 몇 가지 중요한 결과를 지닌다. 첫째로, “영구 기관”은 없다. 둘째로, 엔트로피의 진행 방향은 시간의 진행 방향과 같다.
통계역학에서 엔트로피는 근본적으로 어떠한 계가 정렬될 수 있는 경우의 수를 나타내는 척도이다. 종종 무질서를 나타내는 척도로 간주되기도 한다 (높은 엔트로피는 높은 무질서를 뜻한다). 구체적으로 이 정의에서 엔트로피는 계에 포함되어 있는 개개의 원자와 분자들의 미시적 배열의 경우의수의 로그값에 비례한다. 비례 상수는 볼츠만 상수로 주어진다.
열역학 제2법칙[편집]
열역학 제2법칙은 보통 어떠한 계의 총 엔트로피는 다른 계의 엔트로피가 증가하지 않는 이상 감소하지 않는다는 법칙이다. 이런 이유로 열적으로 고립된 계의 총 엔트로피가 감소하지 않는다. 이것은 차가운 부분에 한 일이 없을 때, 열이 차가운 부분에서 뜨거운 부분으로 흐르지 않는 이유이다. 두번째로 열원(reservoir)으로부터 양의 열에너지를 뽑아서 모두 일로 전환하되, 다른 추가적인 효과를 동반하지 않는 순환과정(cycle)은 존재하지 않는다. 총 일의 생산은 뜨거운 열원에서 차가운 열원으로 열의 이동을 필요로 한다. 따라서 영구 기관은 존재할 수 없다. 마지막으로 어떠한 과정에서 엔트로피 증가가 적다는 것은 그 과정의 에너지 효율이 좋다는 것을 뜻한다.
열역학 제2법칙으로부터 고립계가 아닌 계의 엔트로피는 감소할 수도 있다는 것을 알 수 있다. 예를 들어 에어컨은 방 안의 공기를 차갑게 해준다. 따라서 공기의 엔트로피를 감소시킨다. 방으로 부터 방출되고 에어컨이 작동함에 따라 흡수되는 열은 항상 그 계의 공기의 엔트로피의 감소보다 많은 양의 엔트로피를 만든다. 따라서 전체 계의 총 엔트로피는 열역학 제2법칙에 의하듯 증가한다.
역학에서 열역학의 기본 관계를 사용하여 표현된 제2법칙은 계의 일을 할 수 있는 능력의 한계를 나타낸다. 가역과정에서 미소 열 을 흡수한 온도가 T인 계의 엔트로피 변화는 로 주어진다.
통계역학은 엔트로피가 확률에 의해 지배받는 요소라는 것을 입증한다. 따라서 무질서의 감소가 닫힌계 안에서도 일어날 수 있다. 그러나 이것이 나타날 확률은 매우 작기 때문에 이러한 현상이 나타나더라도 계의 매우 적은 입자들에만 영향을 미치는 일시적인 감소이다.
정의와 설명[편집]
열역학적 엔트로피는 미시적 세계가 고려되는 통계열역학적 관점에서 일반적으로 정의된다. 이와는 다르게 엔트로피는 또한 분자 사이의 상호 작용을 고려하지 않은 대신 개개의 분자의 특성이 전체의 평균 특성을 가지는 매우 많은 분자들의 집합이 운동하는 계를 고려하는 고전열역학적 관점에서 정의될 수 있다. 역사적으로 엔트로피는 고전열역학적으로 먼저 정의되었고 최근에 비평형열역학으로 발전되었다.
통계열역학[편집]
통계 역학적 엔트로피는 불확실성을 나타내는 개념이다. 주어진 거시적 변수들에 대해 엔트로피는 계가 가능한 미시상태에 퍼져있을 확률의 정도를 알려준다. 측정 가능한 평균적인 양들에 의해 결정되는 거시상태와는 반대로, 미시상태는 계의 모든 분자들의 위치와 속도를 포함한 모든 분자의 세부 사항을 명시한다. 많은 가능한 상태들을 지닌 계는 높은 엔트로피를 갖는다.
좀 더 자세히, 엔트로피는 상태밀도의 측정값에 로그를 취한 값이다.
여기서 kB는 볼츠만 상수로써, 그 값은 1.380 6504(24)×10−23 J K-1이다. 합은 계가 가질 수 있는 모든 미시상태를 더한 것이고 Pi는 계의 상태가 i번째 미시상태일 확률을 나타낸다. 거의 모든 실질적인 사용을 위해 이것은 엔트로피의 기본 정의로써 사용될 수 있다. 왜냐하면 S에 대한 다른 정의들은 모두 이 정의에서 유도될 수 있기 때문이다.
통계열역학의 기본 가정 혹은 통계역학의 기본 공준으로 불리는 어떠한 미시상태든지 모두 같은 확률을 같는다는 가정은 (다시 말해, Ω를 미시상태의 개수라고 할 때 Pi=1/Ω이다.) 보통 평형 상태인 고립계에서 성립함을 알 수 있다. 따라서 위의 식은 다음과 같이 변한다.
열역학에서 특정한 부피, 입자의 개수, 내부 에너지를 가진 계는 유일하다 (작은 바른틀 앙상블).
본질적으로 엔트로피의 표현에 대한 가장 일반적인 방식은 계에 대한 우리의 불확실성을 측정한 것이다. 계가 열역학적 평형상태일 때 우리는 보존되는 변수들을 제외한 모든 초기 조건에 관한 정보를 잃었기 때문에 엔트로피는 최대이다. 엔트로피가 최대이면 우리의 계에 대한 특성에 관한 무지 또한 최대이다.
고전열역학[편집]
클라우지우스 정리에 의하면, 가역과정에서 다음이 성립한다.
이것은 선적분인 가 경로에 대하여 독립적이라는 것이다.
따라서 우리는 엔트로피라고 부르는 상태함수인 S를 다음을 만족하는 것으로 정의할 수 있다.
이것을 적분하여 우리는 엔트로피의 값이 아닌 엔트로피의 변화된 양만을 알아낼 수 있다. 값을 정확히 알기 위해서는 절대 영도에서의 완전 결정의 S의 값이 0이라는 법칙인 열역학 제3법칙을 이용하여야 한다.
From a macroscopic perspective, in classical thermodynamics the entropy is interpreted as a state function of a thermodynamic system: that is, a property depending only on the current state of the system, independent of how that state came to be achieved. The state function has the important property that, when multiplied by a reference temperature, it can be understood as a measure of the amount of energy in a physical system that cannot be used to do thermodynamic work; i.e., work mediated by thermal energy.[출처 필요] More precisely, in any process where the system gives up energy ΔE, and its entropy falls by ΔS, a quantity at least TR ΔS of that energy must be given up to the system's surroundings as unusable heat (TR is the temperature of the system's external surroundings). Otherwise the process will not go forward. In classical thermodynamics, the entropy of a system is defined only if it is in thermodynamic equilibrium. 거시적인 관점에서 고전열역학에서의 엔트로피는 열역학적 계에서의 상태함수로 나타내어진다. 다시 말해,
In a thermodynamic system, pressure, density, and temperature tend to become uniform over time because this equilibrium state has higher probability (more possible combinations of microstates) than any other; see statistical mechanics. In the ice melting example, the difference in temperature between a warm room (the surroundings) and cold glass of ice and water (the system and not part of the room), begins to be equalized as portions of the thermal energy from the warm surroundings spread to the cooler system of ice and water.
Over time the temperature of the glass and its contents and the temperature of the room become equal. The entropy of the room has decreased as some of its energy has been dispersed to the ice and water. However, as calculated in the example, the entropy of the system of ice and water has increased more than the entropy of the surrounding room has decreased. In an isolated system such as the room and ice water taken together, the dispersal of energy from warmer to cooler always results in a net increase in entropy. Thus, when the "universe" of the room and ice water system has reached a temperature equilibrium, the entropy change from the initial state is at a maximum. The entropy of the thermodynamic system is a measure of how far the equalization has progressed.
A special case of entropy increase, the entropy of mixing, occurs when two or more different substances are mixed. If the substances are at the same temperature and pressure, there will be no net exchange of heat or work - the entropy change will be entirely due to the mixing of the different substances. At a statistical mechanical level, this results due to the change in available volume per particle with mixing.[2]
History[편집]

The first law of thermodynamics, formalized based on the heat-friction experiments of James Joule in 1843, deals with the concept of energy, which is conserved in all processes; the first law, however, lacks in its ability to quantify the effects of friction and dissipation.
Entropy began with the work of French mathematician Lazare Carnot who in his 1803 paper Fundamental Principles of Equilibrium and Movement proposed that in any machine the accelerations and shocks of the moving parts all represent losses of moment of activity. In other words, in any natural process there exists an inherent tendency towards the dissipation of useful energy. Building on this work, in 1824 Lazare's son Sadi Carnot published Reflections on the Motive Power of Fire in which he set forth the view that in all heat-engines whenever "caloric", or what is now known as heat, falls through a temperature difference, that work or motive power can be produced from the actions of the "fall of caloric" between a hot and cold body. This was an early insight into the second law of thermodynamics.[3]
Carnot based his views of heat partially on the early 18th century "Newtonian hypothesis" that both heat and light were types of indestructible forms of matter, which are attracted and repelled by other matter, and partially on the contemporary views of Count Rumford who showed in 1789 that heat could be created by friction as when cannon bores are machined.[4] Accordingly, Carnot reasoned that if the body of the working substance, such as a body of steam, is brought back to its original state (temperature and pressure) at the end of a complete engine cycle, that "no change occurs in the condition of the working body". This latter comment was amended in his foot notes, and it was this comment that led to the development of entropy.[출처 필요]
In the 1850s and 1860s, German physicist Rudolf Clausius gravely objected to this latter supposition, i.e. that no change occurs in the working body, and gave this "change" a mathematical interpretation by questioning the nature of the inherent loss of usable heat when work is done, e.g. heat produced by friction.[5] Clausius described entropy as the transformation-content, i.e. dissipative energy use, of a thermodynamic system or working body of chemical species during a change of state.[5] This was in contrast to earlier views, based on the theories of Isaac Newton, that heat was an indestructible particle that had mass.
Later, scientists such as Ludwig Boltzmann, Josiah Willard Gibbs, and James Clerk Maxwell gave entropy a statistical basis. In 1877, Boltzmann visualized a probabilistic way to measure the entropy of an ensemble of ideal gas particles, in which he defined entropy to be proportional to the logarithm of the number of microstates such a gas could occupy. Henceforth, the essential problem in statistical thermodynamics, i.e. according to Erwin Schrödinger, has been to determine the distribution of a given amount of energy E over N identical systems. Carathéodory linked entropy with a mathematical definition of irreversibility, in terms of trajectories and integrability.
Consequences and applications[편집]
The arrow of time[편집]
Entropy is the only quantity in the physical sciences that seems to imply a particular direction of progress, sometimes called an arrow of time. As time progresses, the second law of thermodynamics states that the entropy of an isolated system never decreases. Hence, from this perspective, entropy measurement is thought of as a kind of clock.
The fundamental thermodynamic relation[편집]
The entropy of a system depends on its internal energy and the external parameters, such as the volume. In the thermodynamic limit this fact leads to an equation relating the change in the internal energy to changes in the entropy and the external parameters. This relation is known as the fundamental thermodynamic relation. If the volume is the only external parameter, this relation is:
Since the internal energy is fixed when one specifies the entropy and the volume, this relation is valid even if the change from one state of thermal equilibrium to another with infinitesimally larger entropy and volume happens in a non-quasistatic way (so during this change the system may be very far out of thermal equilibrium and then the entropy, pressure and temperature may not exist).
The fundamental thermodynamic relation implies many thermodynamic identities that are valid in general, independent of the microscopic details of the system. Important examples are the Maxwell relations and the relations between heat capacities.
Entropy in chemical thermodynamics[편집]
Thermodynamic entropy is central in chemical thermodynamics, enabling changes to be quantified and the outcome of reactions predicted. The second law of thermodynamics states that entropy in an isolated system, the combination of a subsystem under study and its surroundings, increases during all spontaneous chemical and physical processes. The Clausius equation of δqrev/T = ΔS introduces the measurement of entropy change, ΔS. Entropy change describes the direction and quantifies the magnitude of simple changes such as heat transfer between systems – always from hotter to cooler spontaneously.[6]
The thermodynamic entropy therefore has the dimension of energy divided by temperature, and the unit joule per kelvin (J/K) in the International System of Units (SI).
Thermodynamic entropy is an extensive property, meaning that it scales with the size or extent of a system. In many processes it is useful to specify the entropy as an intensive property independent of the size, as a specific entropy characteristic of the type of system studied. Specific entropy may be expressed relative to a unit of mass, typically the kilogram (unit: Jkg-1K-1). Alternatively, in chemistry, it is also referred to one mole of substance, in which case it is called the molar entropy with a unit of Jmol-1K-1.
Thus, when one mole of substance at 0K is warmed by its surroundings to 298K, the sum of the incremental values of qrev/T constitute each element's or compound's standard molar entropy, a fundamental physical property and an indicator of the amount of energy stored by a substance at 298K.[7][8] Entropy change also measures the mixing of substances as a summation of their relative quantities in the final mixture.[9]
Entropy is equally essential in predicting the extent and direction of complex chemical reactions. For such applications, ΔS must be incorporated in an expression that includes both the system and its surroundings, ΔSuniverse = ΔSsurroundings + ΔS system. This expression becomes, via some steps, the Gibbs free energy equation for reactants and products in the system: ΔG [the Gibbs free energy change of the system] = ΔH [the enthalpy change] −T ΔS [the entropy change].[7]
Entropy change[편집]
When an ideal gas undergoes a change, its entropy may also change. For cases where the specific heat doesn't change and either volume, pressure or temperature is also constant, the change in entropy can be easily calculated.[10]
When specific heat and volume are constant, the change in entropy is given by:
- .
When specific heat and pressure are constant, the change in entropy is given by:
- .
When specific heat and temperature are constant, the change in entropy is given by:
- .
In these equations is the specific heat at constant volume, is the specific heat at constant pressure, is the ideal gas constant, and is the number of moles of gas.
For some other transformations, not all of these properties (specific heat, volume, pressure or temperature) are constant. In these cases, for only 1 mole of an ideal gas, the change in entropy can be given by[11] either:
- or
- .
Entropy balance equation for open systems[편집]
In chemical engineering, the principles of thermodynamics are commonly applied to "open systems", i.e. those in which heat, work, and mass flow across the system boundary. In a system in which there are flows of both heat () and work, i.e. (shaft work) and P(dV/dt) (pressure-volume work), across the system boundaries, the heat flow, but not the work flow, causes a change in the entropy of the system. This rate of entropy change is where T is the absolute thermodynamic temperature of the system at the point of the heat flow. If, in addition, there are mass flows across the system boundaries, the total entropy of the system will also change due to this convected flow.

To derive a generalized entropy balanced equation, we start with the general balance equation for the change in any extensive quantity Θ in a thermodynamic system, a quantity that may be either conserved, such as energy, or non-conserved, such as entropy. The basic generic balance expression states that dΘ/dt, i.e. the rate of change of Θ in the system, equals the rate at which Θ enters the system at the boundaries, minus the rate at which Θ leaves the system across the system boundaries, plus the rate at which Θ is generated within the system. Using this generic balance equation, with respect to the rate of change with time of the extensive quantity entropy S, the entropy balance equation for an open thermodynamic system is:[12]
where
- = the net rate of entropy flow due to the flows of mass into and out of the system (where = entropy per unit mass).
- = the rate of entropy flow due to the flow of heat across the system boundary.
- = the rate of internal generation of entropy within the system.
Note, also, that if there are multiple heat flows, the term is to be replaced by where is the heat flow and is the temperature at the jth heat flow port into the system.
Entropy in quantum mechanics (von Neumann entropy)[편집]
| “ | My greatest concern was what to call it. I thought of calling it ‘information’, but the word was overly used, so I decided to call it ‘uncertainty’. When I discussed it with John von Neumann, he had a better idea. Von Neumann told me, ‘You should call it entropy, for two reasons. In the first place your uncertainty function has been used in statistical mechanics under that name, so it already has a name. In the second place, and more important, nobody knows what entropy really is, so in a debate you will always have the advantage. | ” |
— Conversation between Claude Shannon and John von Neumann regarding what name to give to the “measure of uncertainty” or attenuation in phone-line signals [13]
| ||
In quantum statistical mechanics, the concept of entropy was developed by John von Neumann and is generally referred to as "von Neumann entropy", namely .
where is the density matrix and Tr is the trace operator.
This upholds the correspondence principle, because in the classical limit, i.e. whenever the classical notion of probability applies, this expression is equivalent to the familiar classical definition of entropy,
Von Neumann established a rigorous mathematical framework for quantum mechanics with his work Mathematische Grundlagen der Quantenmechanik. He provided in this work a theory of measurement, where the usual notion of wave function collapse is described as an irreversible process (the so called von Neumann or projective measurement). Using this concept, in conjunction with the density matrix he extended the classical concept of entropy into the quantum domain.
Approaches to understanding entropy[편집]
Order and disorder[편집]
Entropy has often been loosely associated with the amount of order, disorder, and/or chaos in a thermodynamic system. The traditional qualitative description of entropy is that it refers to changes in the status quo of the system and is a measure of "molecular disorder" and the amount of wasted energy in a dynamical energy transformation from one state or form to another.[14] In this direction, a number of authors, in recent years, have derived exact entropy formulas to account for and measure disorder and order in atomic and molecular assemblies.[15][16][17][18] One of the simpler entropy order/disorder formulas is that derived in 1984 by thermodynamic physicist Peter Landsberg, which is based on a combination of thermodynamics and information theory arguments. Landsberg argues that when constraints operate on a system, such that it is prevented from entering one or more of its possible or permitted states, as contrasted with its forbidden states, the measure of the total amount of “disorder” in the system is given by the following expression:[17][18]
Similarly, the total amount of "order" in the system is given by:
In which CD is the "disorder" capacity of the system, which is the entropy of the parts contained in the permitted ensemble, CI is the "information" capacity of the system, an expression similar to Shannon's channel capacity, and CO is the "order" capacity of the system.[16]
Energy dispersal[편집]
The concept of entropy can be described qualitatively as a measure of energy dispersal at a specific temperature.[19] Similar terms have been in use from early in the history of classical thermodynamics, and with the development of statistical thermodynamics and quantum theory, entropy changes have been described in terms of the mixing or "spreading" of the total energy of each constituent of a system over its particular quantized energy levels.
Ambiguities in the terms disorder and chaos, which usually have meanings directly opposed to equilibrium, contribute to widespread confusion and hamper comprehension of entropy for most students.[20] As the second law of thermodynamics shows, in an isolated system internal portions at different temperatures will tend to adjust to a single uniform temperature and thus produce equilibrium. A recently developed educational approach avoids ambiguous terms and describes such spreading out of energy as dispersal, which leads to loss of the differentials required for work even though the total energy remains constant in accordance with the first law of thermodynamics[21] (compare discussion in next section). Physical chemist Peter Atkins, for example, who previously wrote of dispersal leading to a disordered state, now writes that "spontaneous changes are always accompanied by a dispersal of energy".[6][22]
Relating entropy to energy usefulness[편집]
Following on from the above, it is possible (in a thermal context) to regard entropy as an indicator or measure of the effectiveness or usefulness of a particular quantity of energy.[23] This is because energy supplied at a high temperature (i.e. with low entropy) tends to be more useful than the same amount of energy available at room temperature. Mixing a hot parcel of a fluid with a cold one produces a parcel of intermediate temperature, in which the overall increase in entropy represents a “loss” which can never be replaced.
Thus, the fact that the entropy of the universe is steadily increasing, means that its total energy is becoming less useful: eventually, this will lead to the "heat death of the Universe".
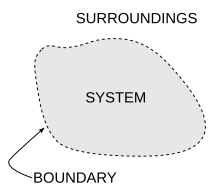
Ice melting example[편집]
The illustration for this article is a classic example in which entropy increases in a small "universe", a thermodynamic system consisting of the "surroundings" (the warm room) and "system" (glass, ice, cold water). In this universe, some thermal energy δQ from the warmer room surroundings (at 298 K or 25 °C) will spread out to the cooler system of ice and water at its constant temperature T of 273 K (0 °C), the melting temperature of ice. The entropy of the system will change by the amount dS = δQ/T, in this example δQ/273 K. (The thermal energy δQ for this process is the energy required to change water from the solid state to the liquid state, and is called the enthalpy of fusion, i.e. the ΔH for ice fusion.) The entropy of the surroundings will change by an amount dS = −δQ/298 K. So in this example, the entropy of the system increases, whereas the entropy of the surroundings decreases.
It is important to realize that the decrease in the entropy of the surrounding room is less than the increase in the entropy of the ice and water: the room temperature of 298 K is larger than 273 K and therefore the ratio, (entropy change), of δQ/298 K for the surroundings is smaller than the ratio (entropy change), of δQ/273 K for the ice+water system. To find the entropy change of our "universe", we add up the entropy changes for its constituents: the surrounding room and the ice+water. The total entropy change is positive; this is always true in spontaneous events in a thermodynamic system and it shows the predictive importance of entropy: the final net entropy after such an event is always greater than was the initial entropy.
As the temperature of the cool water rises to that of the room and the room further cools imperceptibly, the sum of the δQ/T over the continuous range, at many increments, in the initially cool to finally warm water can be found by calculus. The entire miniature "universe", i.e. this thermodynamic system, has increased in entropy. Energy has spontaneously become more dispersed and spread out in that "universe" than when the glass of ice water was introduced and became a "system" within it.
Notice that the system will reach a point where the room, the glass and the contents of the glass will be at the same temperature. In this situation, nothing else can happen: although thermal energy does exist in the room (in fact, the amount of thermal energy is the same as in the beginning, since it is a closed system), it is now unable to do useful work, as there is no longer a temperature gradient. Unless an external event intervenes (thus breaking the definition of a closed system), the room is destined to remain in the same condition for all eternity. Therefore, following the same reasoning but considering the whole universe as our "room", we reach a similar conclusion: that, at a certain point in the distant future, the whole universe will be a uniform, isothermic and inert body of matter, in which there will be no available energy to do work. This condition is known as the "heat death of the Universe".
Entropy and adiabatic accessibility[편집]
A definition of entropy based entirely on the relation of adiabatic accessibility between equilibrium states was given by E.H.Lieb and J. Yngvason in 1999.[24] This approach has several predecessors, including the pioneering work of Constantin Carathéodory from 1909 [25] and the monograph by R. Giles from 1964.[26] In the setting of Lieb and Yngvason one starts by picking, for a unit amount of the substance under consideration, two reference states and such that the latter is adiabatically accessible from the former but not vice versa. Defining the entropies of the reference states to be 0 and 1 respectively the entropy of a state is defined as the largest number such that is adiabatically accessible from a composite state consisting of an amount in the state and a complementary amount, , in the state . A simple but important result within this setting is that entropy is uniquely determined, apart from a choice of unit and an additive constant for each chemical element, by the following properties: It is monotonic with respect to the relation of adiabatic accessibility, additive on composite systems, and extensive under scaling.
Standard textbook definitions[편집]
The following is a list of additional definitions of entropy from a collection of textbooks.
- a measure of energy dispersal at a specific temperature.[6]
- a measure of disorder in the universe or of the availability of the energy in a system to do work.[27]
Interdisciplinary applications of entropy[편집]
Although the concept of entropy was originally a thermodynamic construct, it has been adapted in other fields of study, including information theory, psychodynamics, thermoeconomics, and evolution.[16][28][29]
Thermodynamic and statistical mechanics concepts[편집]
- Entropy unit - a non-S.I. unit of thermodynamic entropy, usually denoted "e.u." and equal to one calorie per Kelvin per mole, or 4.184 Joules per Kelvin per mole.[30]
- Gibbs entropy - the usual statistical mechanical entropy of a thermodynamic system.
- Boltzmann entropy - a type of Gibbs entropy, which neglects internal statistical correlations in the overall particle distribution.
- Tsallis entropy - a generalization of the standard Boltzmann-Gibbs entropy.
- Standard molar entropy - is the entropy content of one mole of substance, under conditions of standard temperature and pressure.
- Residual entropy - the entropy present after a substance is cooled arbitrarily close to absolute zero.
- Entropy of mixing - the change in the entropy when two different chemical substances or components are mixed.
- Loop entropy - is the entropy lost upon bringing together two residues of a polymer within a prescribed distance.
- Conformational entropy - is the entropy associated with the physical arrangement of a polymer chain that assumes a compact or globular state in solution.
- Entropic force - a microscopic force or reaction tendency related to system organization changes, molecular frictional considerations, and statistical variations.
- Free entropy - an entropic thermodynamic potential analogous to the free energy.
- Entropic explosion – an explosion in which the reactants undergo a large change in volume without releasing a large amount of heat.
- Entropy change – a change in entropy dS between two equilibrium states is given by the heat transferred dQrev divided by the absolute temperature T of the system in this interval.[31]
- Sackur-Tetrode entropy - the entropy of a monatomic classical ideal gas determined via quantum considerations.
Entropy and life[편집]
For nearly a century and a half, beginning with Clausius' 1863 memoir "On the Concentration of Rays of Heat and Light, and on the Limits of its Action", much writing and research has been devoted to the relationship between thermodynamic entropy and the evolution of life. The argument that life feeds on negative entropy or negentropy as asserted in the 1944 book What is Life? by physicist Erwin Schrödinger served as a further stimulus to this research. Recent writings have used the concept of Gibbs free energy to elaborate on this issue.[32]
In the 1982 textbook Principles of Biochemistry by American biochemist Albert Lehninger, for example, it is argued that the "order" produced within cells as they grow and divide is more than compensated for by the "disorder" they create in their surroundings in the course of growth and division. In short, according to Lehninger, "living organisms preserve their internal order by taking from their surroundings free energy, in the form of nutrients or sunlight, and returning to their surroundings an equal amount of energy as heat and entropy."[33]
Evolution-related concepts:
- Negentropy - a shorthand colloquial phrase for negative entropy.[34]
- Ectropy - a measure of the tendency of a dynamical system to do useful work and grow more organized.[14]
- Extropy – a metaphorical term defining the extent of a living or organizational system's intelligence, functional order, vitality, energy, life, experience, and capacity and drive for improvement and growth.
- Ecological entropy - a measure of biodiversity in the study of biological ecology.
In a study titled “Natural selection for least action” published in the Proceedings of The Royal Society A., Ville Kaila and Arto Annila of the University of Helsinki describe how the second law of thermodynamics can be written as an equation of motion to describe evolution, showing how natural selection and the principle of least action can be connected by expressing natural selection in terms of chemical thermodynamics. In this view, evolution explores possible paths to level differences in energy densities and so increase entropy most rapidly. Thus, an organism serves as an energy transfer mechanism, and beneficial mutations allow successive organisms to transfer more energy within their environment.[35]
Cosmology[편집]
Since a finite universe is an isolated system then, by the Second Law of Thermodynamics, its total entropy is constantly increasing. It has been speculated, since the 19th century, that the universe is fated to a heat death in which all the energy ends up as a homogeneous distribution of thermal energy, so that no more work can be extracted from any source.
If the universe can be considered to have generally increasing entropy, then—as Roger Penrose has pointed out—gravity plays an important role in the increase because gravity causes dispersed matter to accumulate into stars, which collapse eventually into black holes. The entropy of a black hole is proportional to the surface area of the black hole's event horizon.[36] Jacob Bekenstein and Stephen Hawking have shown that black holes have the maximum possible entropy of any object of equal size. This makes them likely end points of all entropy-increasing processes, if they are totally effective matter and energy traps. Hawking has, however, recently changed his stance on this aspect.[출처 필요]
The role of entropy in cosmology remains a controversial subject. Recent work has cast some doubt on the heat death hypothesis and the applicability of any simple thermodynamic model to the universe in general. Although entropy does increase in the model of an expanding universe, the maximum possible entropy rises much more rapidly, moving the universe further from the heat death with time, not closer. This results in an "entropy gap" pushing the system further away from the posited heat death equilibrium.[37] Other complicating factors, such as the energy density of the vacuum and macroscopic quantum effects, are difficult to reconcile with thermodynamical models, making any predictions of large-scale thermodynamics extremely difficult.[38]
The entropy gap is widely believed to have been originally opened up by the early rapid exponential expansion of the universe.
Information theory[편집]
In information theory, entropy is the measure of the amount of information that is missing before reception and is sometimes referred to as Shannon entropy.[39] Shannon entropy is a broad and general concept which finds applications in information theory as well as thermodynamics. It was originally devised by Claude Shannon in 1948 to study the amount of information in a transmitted message. The definition of the information entropy is, however, quite general, and is expressed in terms of a discrete set of probabilities :
In the case of transmitted messages, these probabilities were the probabilities that a particular message was actually transmitted, and the entropy of the message system was a measure of how much information was in the message. For the case of equal probabilities (i.e. each message is equally probable), the Shannon entropy (in bits) is just the number of yes/no questions needed to determine the content of the message.[40]
The question of the link between information entropy and thermodynamic entropy is a debated topic. While most authors argue that there is a link between the two,[41][42][43] a few argue that they have nothing to do with each other.[40][44]
The expressions for the two entropies are very similar. The information entropy H for equal probabilities is
where k is a constant which determines the units of entropy. For example, if the units are bits, then k = 1/ln(2). The thermodynamic entropy S, from a statistical mechanical point of view, was first expressed by Boltzmann:
where p is the probability of a system being in a particular microstate, given that it is in a particular macrostate, and is Boltzmann's constant. It can be seen that one may think of the thermodynamic entropy as Boltzmann's constant, divided by log(2), times the number of yes/no questions that must be asked in order to determine the microstate of the system, given that we know the macrostate. The link between thermodynamic and information entropy was developed in a series of papers by Edwin Jaynes beginning in 1957.[45]
There are many ways of demonstrating the equivalence of "information entropy" and "physics entropy", that is, the equivalence of "Shannon entropy" and "Boltzmann entropy". Nevertheless, some authors argue for dropping the word entropy for the H function of information theory and using Shannon's other term "uncertainty" instead.[46]
Mathematics[편집]
- Kolmogorov-Sinai entropy - a mathematical type of entropy in dynamical systems related to measures of partitions.[40]
- Relative entropy - is a natural distance measure from a "true" probability distribution P to an arbitrary probability distribution Q.
- Rényi entropy - a generalized entropy measure for fractal systems.
- Topological entropy - a way of defining entropy in an iterated function map in ergodic theory.
- Volume entropy - a Riemannian invariant measuring the exponential rate of volume growth.
Sociology[편집]
The concept of entropy has also entered the domain of sociology, generally as a metaphor for chaos, disorder or dissipation of energy, rather than as a direct measure of thermodynamic or information entropy:
- Corporate entropy - energy waste as red tape and business team inefficiency, i.e. energy lost to waste.[47] (This definition is comparable to von Clausewitz's concept of friction in war.)
- Economic entropy – a semi-quantitative measure of the irrevocable dissipation and degradation of natural materials and available energy with respect to economic activity.[42][48]
- Entropology – the study or discussion of entropy or the name sometimes given to thermodynamics without differential equations.[49][50]
- Psychological entropy - the distribution of energy in the psyche, which tends to seek equilibrium or balance among all the structures of the psyche.[51]
- Social entropy – a measure of social system structure, having both theoretical and statistical interpretations, i.e. society (macrosocietal variables) measured in terms of how the individual functions in society (microsocietal variables); also related to social equilibrium.[52]
See also[편집]
- Autocatalytic reactions and order creation
- Brownian ratchet
- Chaos theory
- Clausius–Duhem inequality
- Configuration entropy
- Departure function
- Entropy rate
- Geometrical frustration
- Laws of thermodynamics
- Multiplicity function
- Non-equilibrium thermodynamics
- Orders of magnitude (entropy)
- Randomness
- Stirling's formula
- Thermodynamic databases for pure substances
- Thermodynamic potential
- Baryogenesis
Notes[편집]
- ↑ In complex systems of molecules, such as at the critical point of water or when salt is added to an ice-water mixture, entropy can either increase or decrease depending on system parameters, such as temperature and pressure. For example, if the spontaneous crystallization of a supercooled liquid takes place under adiabatic conditions the entropy of the resulting crystal will be greater than that of the supercooled liquid (Denbigh, K. (1982). The Principles of Chemical Equilibrium, 4th Ed.). In general, however, when ice melts, the entropy of the two adjoined systems, the hot and cold bodies, increases. Here are some further tutorials: Ice-melting – JCE example; Ice-melting and Entropy Change – example; Ice-melting and Entropy Change – discussions
References[편집]
- ↑ Clausius, Rudolf (1862). Communicated to the Naturforschende Gesellschaft of Zurich, January 27, 1862; published in the Vierteljahrschrift of this Society, vol. vii. P. 48; in Poggendorff’s Annalen, May 1862, vol. cxvi. p. 73; in the Philosophical Magazine, S. 4. vol. xxiv. pp. 81, 201; and in the Journal des Mathematiques of Paris, S. 2. vol. vii. P. 209.
- ↑ Ben-Naim, Arieh, On the So-Called Gibbs Paradox, and on the Real Paradox, Entropy, 9, 132-136, 2007 Link
- ↑ “Carnot, Sadi (1796-1832)”. Wolfram Research. 2007. 2010년 2월 24일에 확인함.
- ↑ McCulloch, Richard, S. (1876). 《Treatise on the Mechanical Theory of Heat and its Applications to the Steam-Engine, etc.》. D. Van Nostrand.
- ↑ 가 나 Clausius, Rudolf (1850). 《On the Motive Power of Heat, and on the Laws which can be deduced from it for the Theory of Heat》. Poggendorff's Annalen der Physick, LXXIX (Dover Reprint). ISBN 0-486-59065-8.
- ↑ 가 나 다 Atkins, Peter; Julio De Paula (2006). 《Physical Chemistry, 8th edition》. Oxford University Press. ISBN 0-19-870072-5.
- ↑ 가 나 Moore, J. W.; C. L. Stanistski, P. C. Jurs (2005). 《Chemistry, The Molecular Science,》. Brooks Cole. ISBN 0-534-42201-2.
- ↑ Jungermann, A.H. (2006). “Entropy and the Shelf Model: A Quantum Physical Approach to a Physical Property”. 《Journal of Chemical Education》 83 (11): 1686–1694. Bibcode:2006JChEd..83.1686J. doi:10.1021/ed083p1686.
- ↑ Levine, I. N. (2002). 《Physical Chemistry, 5th edition》. McGraw-Hill. ISBN 0-07-231808-2.
- ↑ http://www.grc.nasa.gov/WWW/k-12/Numbers/Math/Mathematical_Thinking/ideal_gases_under_constant.htm
- ↑ http://www.grc.nasa.gov/WWW/K-12/airplane/entropy.html
- ↑ Sandler, Stanley, I. (1989). 《Chemical and Engineering Thermodynamics》. John Wiley & Sons. ISBN 0-471-83050-X.
- ↑ M. Tribus, E.C. McIrvine, Energy and information, Scientific American, 224 (September 1971), 178–184.
- ↑ 가 나 Haddad, Wassim M.; Chellaboina, VijaySekhar; Nersesov, Sergey G. (2005). 《Thermodynamics - A Dynamical Systems Approach》. Princeton University Press. ISBN 0-691-12327-6.
- ↑ Callen, Herbert, B (2001). 《Thermodynamics and an Introduction to Thermostatistics, 2nd Ed.》. John Wiley and Sons. ISBN 0-471-86256-8.
- ↑ 가 나 다 Brooks, Daniel, R.; Wiley, E.O. (1988). 《Evolution as Entropy– Towards a Unified Theory of Biology》. University of Chicago Press. ISBN 0-226-07574-5.
- ↑ 가 나 Landsberg, P.T. (1984). “Is Equilibrium always an Entropy Maximum?” J. Stat. Physics 35: 159-69.
- ↑ 가 나 Landsberg, P.T. (1984). “Can Entropy and “Order” Increase Together?” Physics Letters 102A:171-173
- ↑ Frank L. Lambert, A Student’s Approach to the Second Law and Entropy
- ↑ Carson, E. M. and J. R. Watson (Department of Educational and Professional Studies, Kings College, London), Undergraduate students' understandings of entropy and Gibbs Free energy, University Chemistry Education - 2002 Papers, Royal Society of Chemistry.
- ↑ Frank L. Lambert, JCE 2002 (79) 187 [Feb] Disorder—A Cracked Crutch for Supporting Entropy Discussions
- ↑ Atkins, Peter (1984). 《The Second Law》. Scientific American Library. ISBN 0-7167-5004-X.
- ↑ Sandra Saary (Head of Science, Latifa Girls’ School, Dubai) (1993년 2월 23일). “Book Review of "A Science Miscellany"”. 《Khaleej Times》 (Galadari Press, UAE): XI.
- ↑ Elliott H. Lieb, Jakob Yngvason: The Physics and Mathematics of the Second Law of Thermodynamics, Phys. Rep. 310, 1-96 (1999)
- ↑ Constantin Carathéodory: Untersuchungen über die Grundlagen der Thermodynamik, Math. Ann., 67:355–386, 1909
- ↑ Robin Giles: Mathematical Foundations of Thermodynamics", Pergamon, Oxford 1964
- ↑ Gribbin's Encyclopedia of Particle Physics, 2000
- ↑ Avery, John (2003). 《Information Theory and Evolution》. World Scientific. ISBN 981-238-399-9.
- ↑ Yockey, Hubert, P. (2005). 《Information Theory, Evolution, and the Origin of Life.》. Cambridge University Press. ISBN 0-521-80293-8.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). 온라인 수정 버전: (2006–) "Entropy unit". doi 10.1351/goldbook.E02151
- ↑ Serway, Raymond, A. (1992). 《Physics for Scientists and Engineers》. Saunders Golden Subburst Series. ISBN 0-03-096026-6.
- ↑ Higgs, P. G., & Pudritz, R. E. (2009). “A thermodynamic basis for prebiotic amino acid synthesis and the nature of the first genetic code" Accepted for publication in Astrobiology
- ↑ Lehninger, Albert (1993). 《Principles of Biochemistry, 2nd Ed.》. Worth Publishers. ISBN 0-87901-711-2.
- ↑ Schrödinger, Erwin (1944). 《What is Life - the Physical Aspect of the Living Cell》. Cambridge University Press. ISBN 0-521-42708-8.
- ↑ Lisa Zyga (2008년 8월 11일). “Evolution as Described by the Second Law of Thermodynamics”. Physorg.com. 2008년 8월 14일에 확인함.
- ↑ von Baeyer, Christian, H. (2003). 《Information - the New Language of Science》. Harvard University Press. ISBN 0-674-01387-5.Srednicki M (1993년 8월). “Entropy and area”. 《Phys. Rev. Lett.》 71 (5): 666–669. arXiv:hep-th/9303048. Bibcode:1993PhRvL..71..666S. doi:10.1103/PhysRevLett.71.666. PMID 10055336. Callaway DJE (1996년 4월). “Surface tension, hydrophobicity, and black holes: The entropic connection”. 《Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics》 53 (4): 3738–3744. arXiv:cond-mat/9601111. Bibcode:1996PhRvE..53.3738C. doi:10.1103/PhysRevE.53.3738. PMID 9964684.
- ↑ Stenger, Victor J. (2007). 《God: The Failed Hypothesis》. Prometheus Books. ISBN 159-102-481-1.
- ↑ Benjamin Gal-Or (1981, 1983, 1987). 《Cosmology, Physics and Philosophy》. Springer Verlag. ISBN 0387965262.
- ↑ Balian, Roger (2003). Entropy – Protean Concept (PDF). Poincaré Seminar 2: 119-45.
- ↑ 가 나 다 인용 오류:
<ref>태그가 잘못되었습니다;Perplexed라는 이름을 가진 주석에 텍스트가 없습니다 - ↑ Brillouin, Leon (1956). 《Science and Information Theory》. name. ISBN 0-486-43918-6.
- ↑ 가 나 Georgescu-Roegen, Nicholas (1971). 《The Entropy Law and the Economic Process》. Harvard University Press. ISBN 0-674-25781-2.
- ↑ Chen, Jing (2005). 《The Physical Foundation of Economics - an Analytical Thermodynamic Theory》. World Scientific. ISBN 981-256-323-7.
- ↑ Lin, Shu-Kun. (1999). “Diversity and Entropy.” Entropy (Journal), 1[1], 1-3.
- ↑ “Edwin T. Jaynes - Bibliography”. Bayes.wustl.edu. 1998년 3월 2일. 2009년 12월 6일에 확인함.
- ↑ Schneider, Tom, DELILA system (Deoxyribonucleic acid Library Language), (Information Theory Analysis of binding sites), Laboratory of Mathematical Biology, National Cancer Institute, FCRDC Bldg. 469. Rm 144, P.O. Box. B Frederick, MD 21702-1201, USA.
- ↑ DeMarco, Tom; Lister, Timothy (1999). 《Peopleware: Productive Projects and Teams, 2nd. Ed.》. Dorset House Publishing Co. ISBN 0-932633-43-9.
- ↑ Burley, Peter; Foster, John (1994). 《Economics and Thermodynamics – New Perspectives on Economic Analysis》. Kluwer Academic Publishers. ISBN 0-7923-9446-1.
- ↑ Perrot, Pierre (1998). 《A to Z of Thermodynamics》. Oxford University Press. ISBN 0-19-856552-6.
- ↑ Example: "Entropology, not anthropology, should be the word for the discipline that devotes itself to the study of the process of disintegration in its most evolved forms." (In A World on Wane, London, 1961, pg. 397; translated by John Russell of Tristes Tropiques by Claude Lévi-Strauss.)
- ↑ Hall, Calvin S; Nordby, Vernon J. (1999). 《A Primer of Jungian Psychology》. New York: Meridian. ISBN 0-452-01186-8.
- ↑ Bailey, Kenneth, D. (1990). 《Social Entropy Theory》. State University of New York Press. ISBN 0-7914...
|isbn=값 확인 필요: invalid character (도움말).
Further reading[편집]
- Ben-Naim, Arieh (2007). 《Entropy Demystified》. World Scientific. ISBN 981-270-055-2.
- Dugdale, J. S. (1996). 《Entropy and its Physical Meaning》 2판. Taylor and Francis (UK); CRC (US). ISBN 0748405690.
- Fermi, Enrico (1937). 《Thermodynamics》. Prentice Hall. ISBN 0-486-60361-X.
- Gyftopoulos, E.P.; G.P. Beretta (1991, 2005, 2010). 《Thermodynamics. Foundations and Applications》. Dover. ISBN 0-486-43932-1.
- Kroemer, Herbert; Charles Kittel (1980). 《Thermal Physics》 2판. W. H. Freeman Company. ISBN 0-7167-1088-9.
- Penrose, Roger (2005). 《The Road to Reality: A Complete Guide to the Laws of the Universe》. New York: A.A. Knopf. ISBN 0-679-45443-8.
- Reif, F. (1965). 《Fundamentals of statistical and thermal physics》. McGraw-Hill. ISBN 0-07-051800-9.
- Goldstein, Martin; Inge, F (1993). 《The Refrigerator and the Universe》. Harvard University Press. ISBN 0-674-75325-9.
- vonBaeyer; Hans Christian (1998). 《Maxwell's Demon: Why Warmth Disperses and Time Passes》. Random House. ISBN 0-679-43342-2.
- Entropy for beginners
External links[편집]
- Entropy - A Basic Understanding A primer for entropy from a chemical perspective
- Interactive Shockwave Animation on Entropy
- Max Jammer (1973). Dictionary of the History of Ideas: Entropy
- Frank L. Lambert; entropysite.oxy.edu – links to articles including simple introductions to entropy for chemistry students and for general readers.
- Thermodynamics - a chapter from an online textbook
- Entropy on Project PHYSNET
- Entropy - an Open Access journal
- An Intuitive Guide to the Concept of Entropy Arising in Various Sectors of Science - a wikibook on the interpretation of the concept of entropy.













































